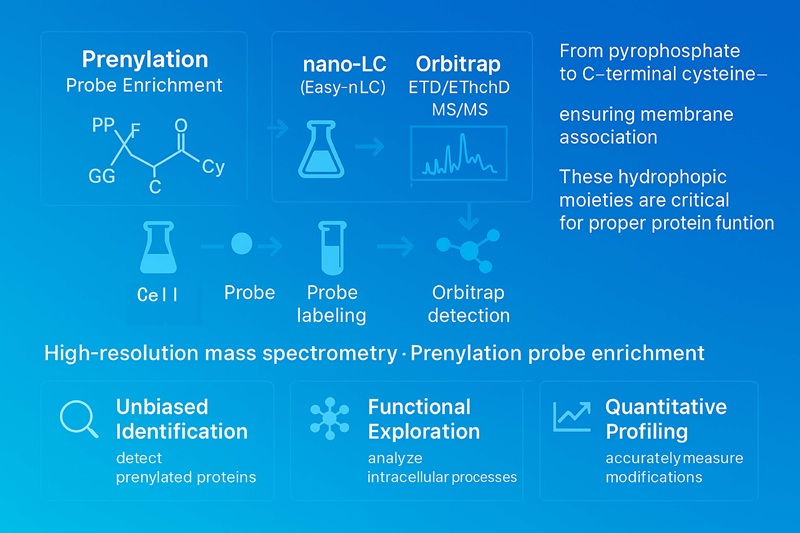
Prenylation Analysis – Systematic LC-MS/MS Characterization of Prenylated Proteins
Prenylation is a process in which hydrophobic isoprenoid groups, such as farnesyl or geranylgeranyl, are covalently attached to specific cysteine residues. This modification plays a key role in protein localization to membranes, signaling pathways, and regulation of the cardiovascular system. Creative Proteomics offers high-precision prenylation research services with a focus on identifying and quantifying prenylated proteins and their modifications.
- Comprehensive detection: Identify and quantify prenylated proteins and specific amino acid modifications.
- Advanced technology: Use of metabolic labeling combined with LC-MS/MS to ensure accuracy and depth of analysis.
- Functional insights: Integration of bioinformatics tools to analyze motifs, pathways, and networks.
- Research impact: Clarify the regulatory roles of prenylation in signaling pathways and provide new insights into the mechanisms and therapeutic targets of cardiovascular disease.
Submit Your Request Now
×
- Define
- What We Provide
- Technology Platform
- Workflow
- Advantages
- Applications
- Demo
- FAQs
- Case
- Publications
- Sample Requirement
What Is Prenylation?
Prenylation is a covalent post-translational modification in which a farnesyl (C15) or geranylgeranyl (C20) group is transferred from its pyrophosphate donor (FPP or GGPP) to a cysteine residue within a C-terminal CAAX motif of target proteins. This process is catalyzed by enzymes such as farnesyltransferase (FTase) or geranylgeranyltransferases (GGTase-I/II). It involves multiple steps including transfer of the prenyl group, proteolytic cleavage of the "AAX" residues, and methylation of the modified cysteine. This increases the protein's hydrophobicity, enabling membrane localization and proper subcellular placement. Prenylation plays crucial roles in cellular signaling, vesicle trafficking, and protein interactions by regulating small GTPases like Ras, Rho, and Rab. Dysregulation of prenylation has been linked to conditions such as cancer, metabolic disorders, and cardiovascular and neurodegenerative diseases[1].
Prenylation Service at Creative Proteomics
Given the biological importance and site-specific nature of prenylation, it is not only a fundamental regulatory mechanism but also a promising target for therapeutic and biotechnological applications. Therefore, identifying prenylated proteins and their sites in cells is essential. We offer comprehensive prenylation proteomics services, including high-resolution mass spectrometry, site-specific identification, quantitative profiling, and advanced bioinformatics analysis. Our services help researchers investigate prenylated proteins systematically, explore their pathway associations, and uncover their clinical and therapeutic significance.
Prenylation Research Platforms

Thermo Fisher Easy-nLC 1000 and Thermo Fisher LTQ Obitrap ETD
(Figure from Thermo Scientific)
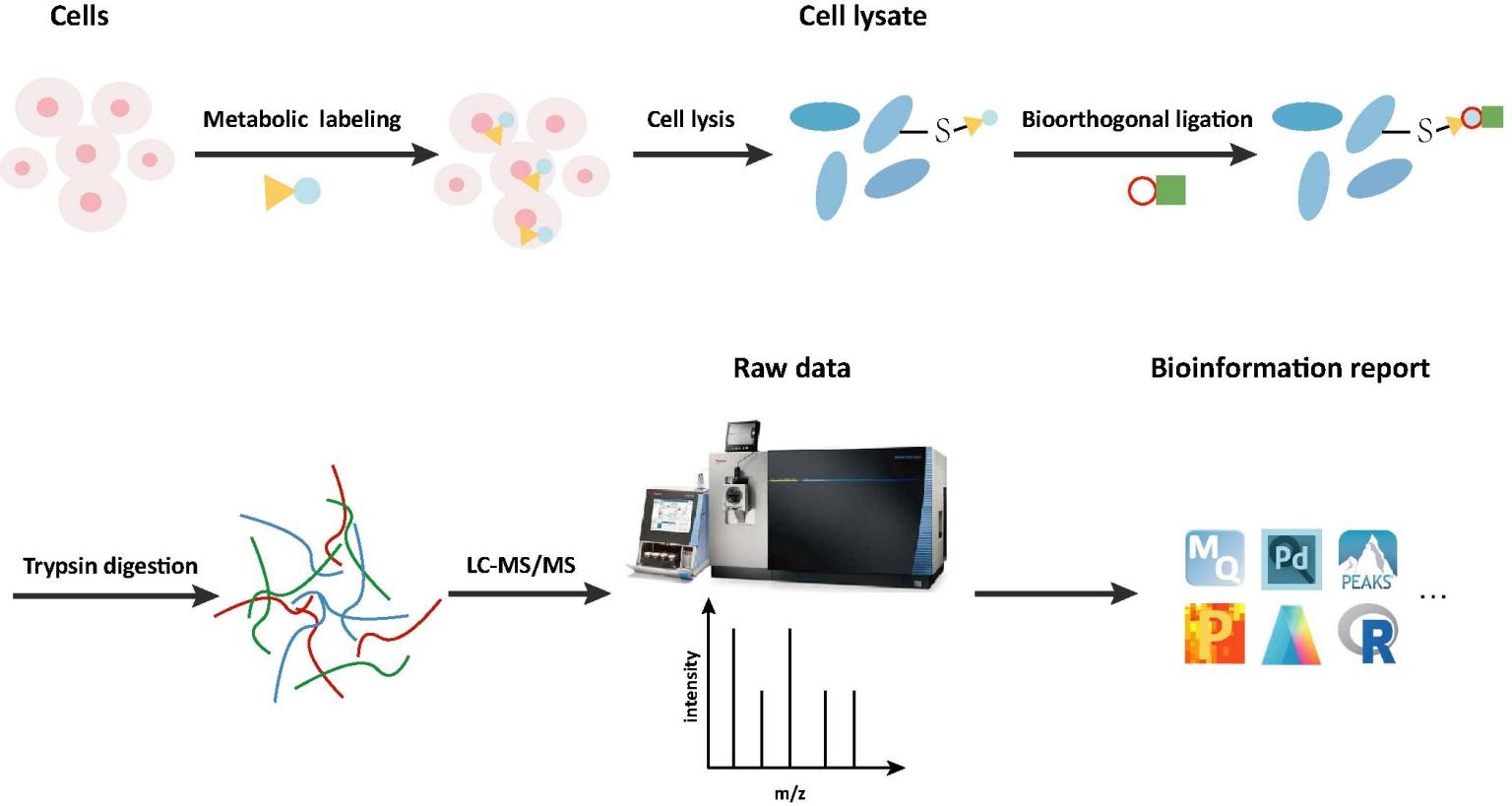
Workflow of Prenylation Analysis
Advantages Our Prenylation Service
- Professional detection and analysis capability: Our experienced research team and well-established techniques ensure reliable results.
- High specificity and accuracy: We use skilled quantification proteomics techniques along with PTMs enrichment methods.
- High stability and reproducibility: Our methods produce consistent and reproducible data across different runs.
- Advanced analytical platforms: We use high-sensitivity mass spectrometry and the latest tools to ensure precise and repeatable results.
- End-to-end support: We provide a full range of services from design to data interpretation, with ongoing technical assistance throughout the research process.
Applications of Prenylation




Cancer
Prenylation affects tumor metabolic reprogramming and epigenetic alterations by regulating histone function and non-histone activity, and can serve as a tumor diagnostic marker and therapeutic target[2].
Metabolic diseases
Protein prenylation plays a critical role in metabolic disorders such as obesity, diabetes, and fatty liver disease by regulating insulin secretion, lipid metabolism, and cellular signaling pathways, highlighting its potential as a therapeutic target and biomarker[3].
Neurodegenerative disease
Abnormal protein phenylation is associated with neurodegenerative processes: regulating the prenylation pathway can provide new targets for the treatment of neurological diseases and the development of biomarkers[4].
Cardiometabolic health
Protein prenylation is closely involved in cardiovascular diseases by modulating endothelial function, vascular inflammation, and smooth muscle cell signaling, thereby influencing processes such as atherosclerosis, hypertension, and cardiac remodeling, and serving as a potential therapeutic target[5].
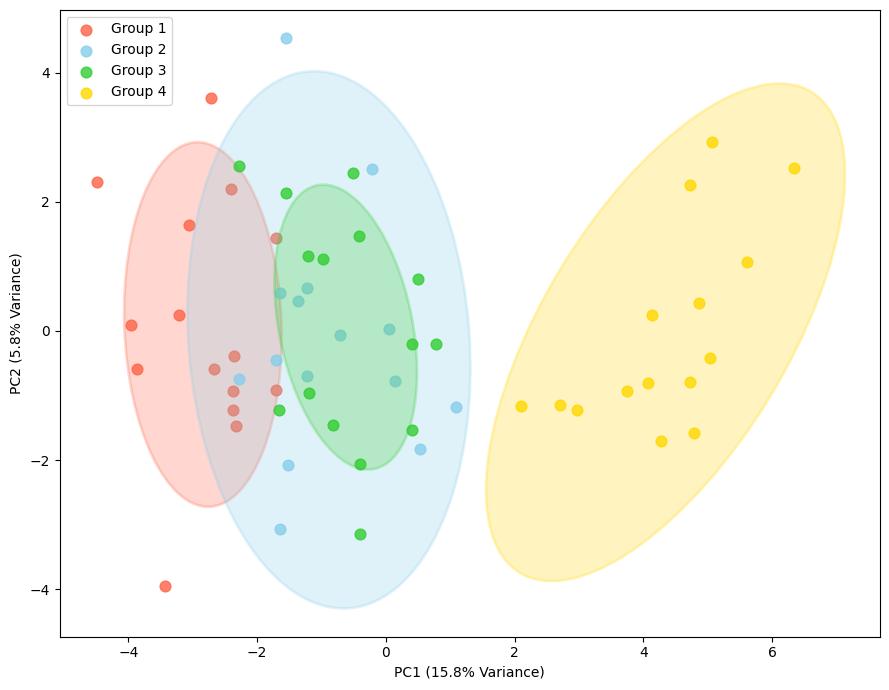
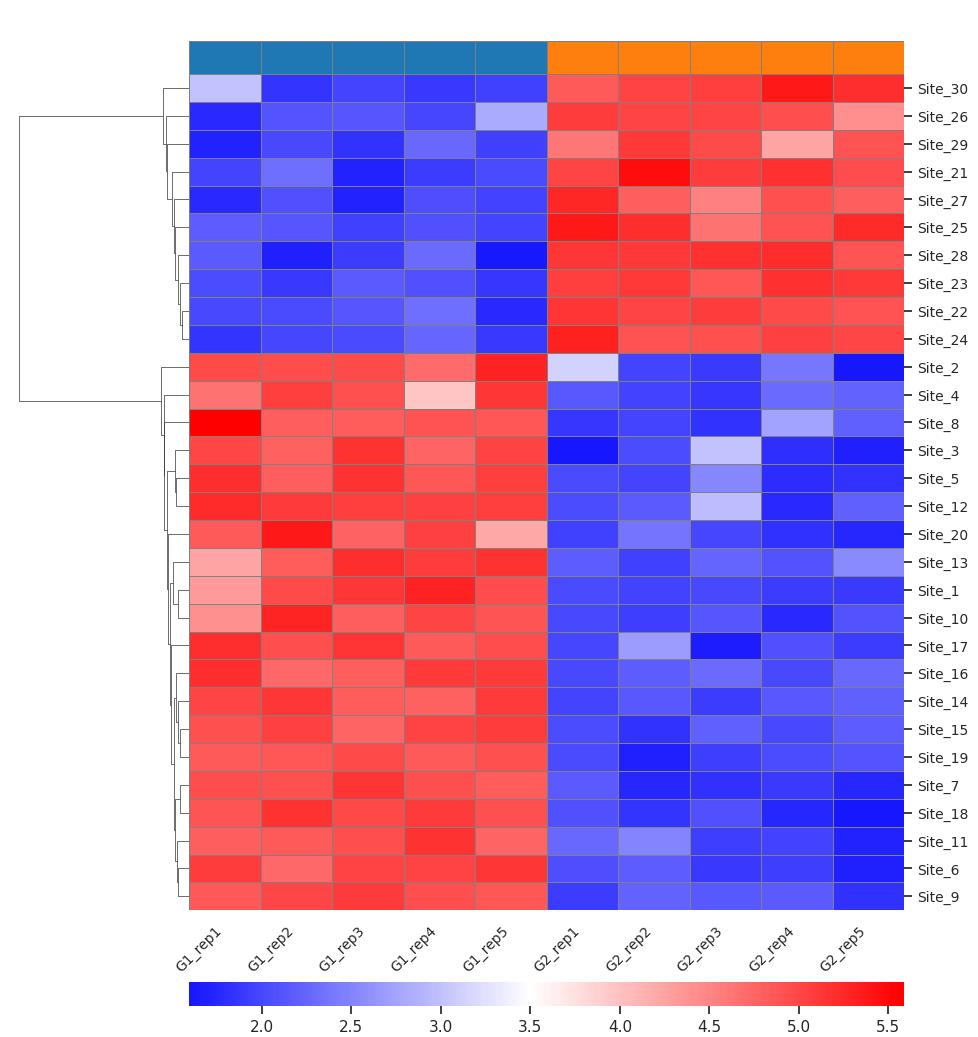
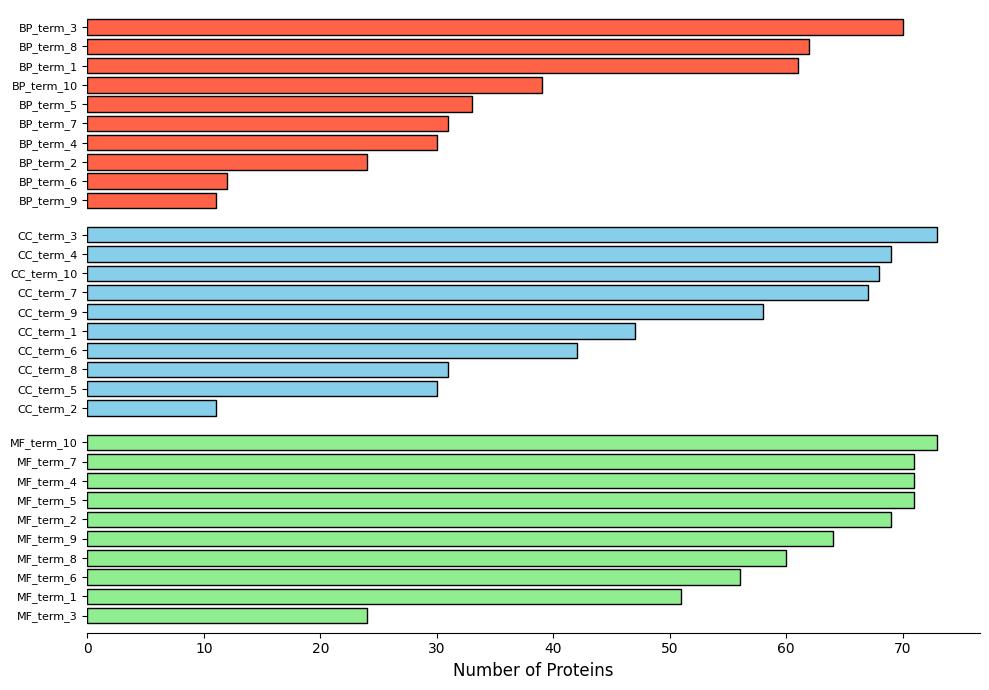
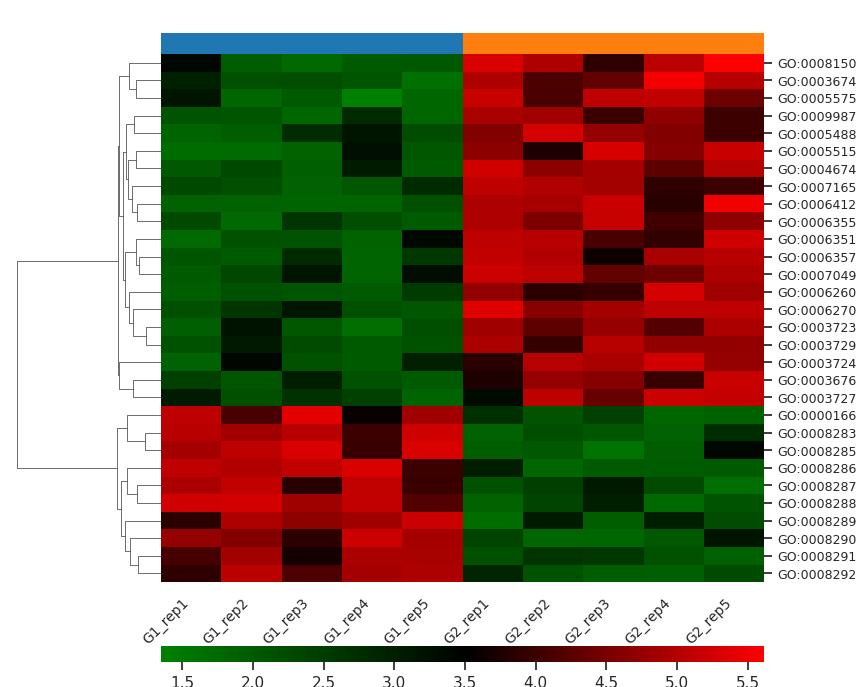
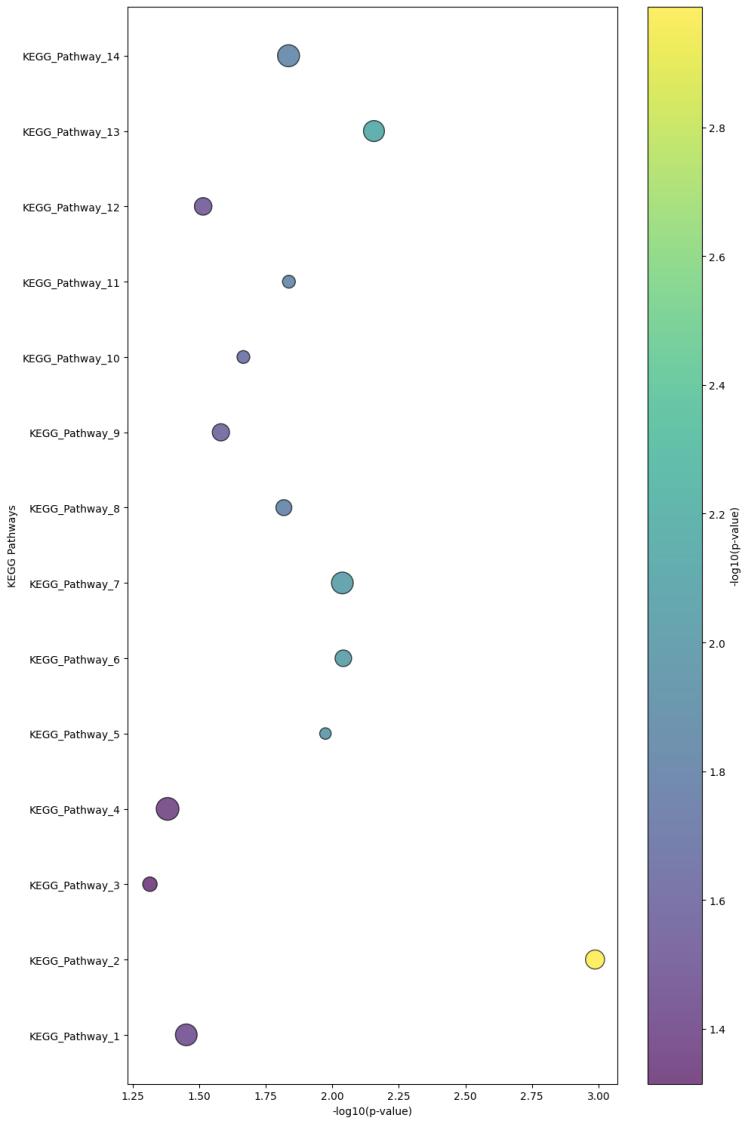
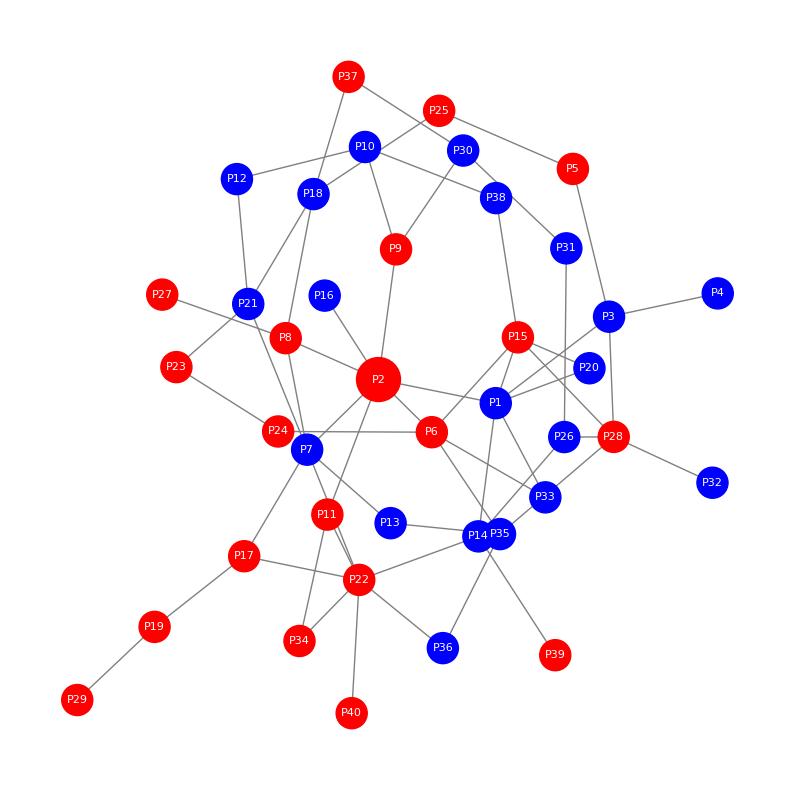
Demo Result of Prenylation Service
FAQs of Prenylation Service
Which sample types are accepted by the service?
We only accept samples of cell. We recommend that customers provide fresh or frozen samples, which should avoid repeated freezing and thawing, and we will be responsible for subsequent protein extraction and pretreatment.
Whether the sample needs pretreatment?
If you need this service, please contact us before cell culture. We will provide the probes for co-incubation with cells and the labeling methods.
Learn about other Q&A.
Case Study

Publications
Here are some publications in Proteomics research from our clients:

- Untargeted proteomics and stage-specific Huntington’s disease reveal biological pathways, and potential protein biomarkers. 2024. https://doi.org/10.21203/rs.3.rs-4508811/v1
- Characterizing the proteome of bullous pemphigoid blister fluid utilizing tandem mass tag labeling coupled with LC–MS/MS. 2022. https://doi.org/10.1007/s00403-021-02253-8
- In vitro assays reveal inherently insecticide-tolerant termite symbionts. 2023. https://doi.org/10.3389/fphys.2023.1134936
- Regulation of cardiac ferroptosis in diabetic human heart failure: uncovering molecular pathways and key targets. 2024. https://doi.org/10.1038/s41420-024-02044-w
- Microglia-mediated synaptic pruning in the nucleus accumbens during adolescence: A preliminary study of the proteomic consequences and putative female-specific pruning target. 2023. https://doi.org/10.1101/2023.05.03.539317
Prenylation Service Sample Requirements
| Sample | Sample Quantity | |
|---|---|---|
| Cell | suspension cell | > 3x108 |
| adherent cell | > 3x108 | |
References
- R. Surana, K., B. Pawar, R., A. Khairnar, R., & K. Mahajan, S. (2024). Protein Prenylation and Their Applications. IntechOpen. doi: 10.5772/intechopen.104700.
- Wang, J., Yao, X., & Huang, J. (2017). New tricks for human farnesyltransferase inhibitor: cancer and beyond. MedChemComm, 8(5), 841–854. https://doi.org/10.1039/c7md00030h.
- Wu, X., Xu, M., Geng, M., Chen, S., Little, P. J., Xu, S., & Weng, J. (2023). Targeting protein modifications in metabolic diseases: molecular mechanisms and targeted therapies. Signal transduction and targeted therapy, 8(1), 220. https://doi.org/10.1038/s41392-023-01439-y
- Jeong, A., Cheng, S., Zhong, R., Bennett, D. A., Bergö, M. O., & Li, L. (2021). Protein farnesylation is upregulated in Alzheimer's human brains and neuron-specific suppression of farnesyltransferase mitigates pathogenic processes in Alzheimer's model mice. Acta neuropathologica communications, 9(1), 129. https://doi.org/10.1186/s40478-021-01231-5
- Shi, W., Chen, Y., Zhang, X., Lou, L., Wang, P., Jiang, R., Liu, S., Sok, T., Zhang, Q., Guo, X., & Yang, J. (2025). Research advances in protein prenylation in cardiovascular diseases. European journal of pharmacology, 1005, 178093. https://doi.org/10.1016/j.ejphar.2025.178093