Ames Test Service | Reliable Mutagenicity Screening for Research & Development
The Ames test is a globally recognized assay for detecting mutagenicity and assessing genetic toxicity in compounds. At Creative Proteomics, we provide a complete suite of Ames test services to help pharmaceutical developers, biotech teams, academic researchers, and CROs identify potential carcinogens early in the development cycle. By integrating both standard and enhanced Ames assays, we ensure accurate, OECD guideline-based results that meet international regulatory requirements.
Problems We Solve for Clients:
- Detect potential carcinogens and mutagens early in discovery.
- Prevent costly late-stage failures by screening compounds up front.
- Meet OECD 471, ICH S2(R1), and EMA/FDA regulatory guidance.
- Reduce false negatives with advanced S9 metabolic activation systems.
Our Key Advantages:
- Full coverage of Salmonella typhimurium and Escherichia coli tester strains.
- Flexible testing options including Micro Ames, Ames MPF, Ames II, and Mini Ames.
- Professional toxicology team with decades of experience.
- Publication-ready reporting with expert interpretation.
- Fast turnaround, global testing facilities, and consistent data quality.
Submit Your Request Now
×
- Accurate Ames Test with Salmonella and E. coli strains
- Enhanced Ames Test with S9 metabolic activation
- Flexible assay platforms: Ames II, MPF, Micro Ames, Mini Ames
- OECD guideline-based results for drug discovery, chemicals, food & environment
- Principle
- Strains
- Services
- Enhanced Test
- Workflow
- Applications
- Why Us
- FAQ
What is the Ames Test?
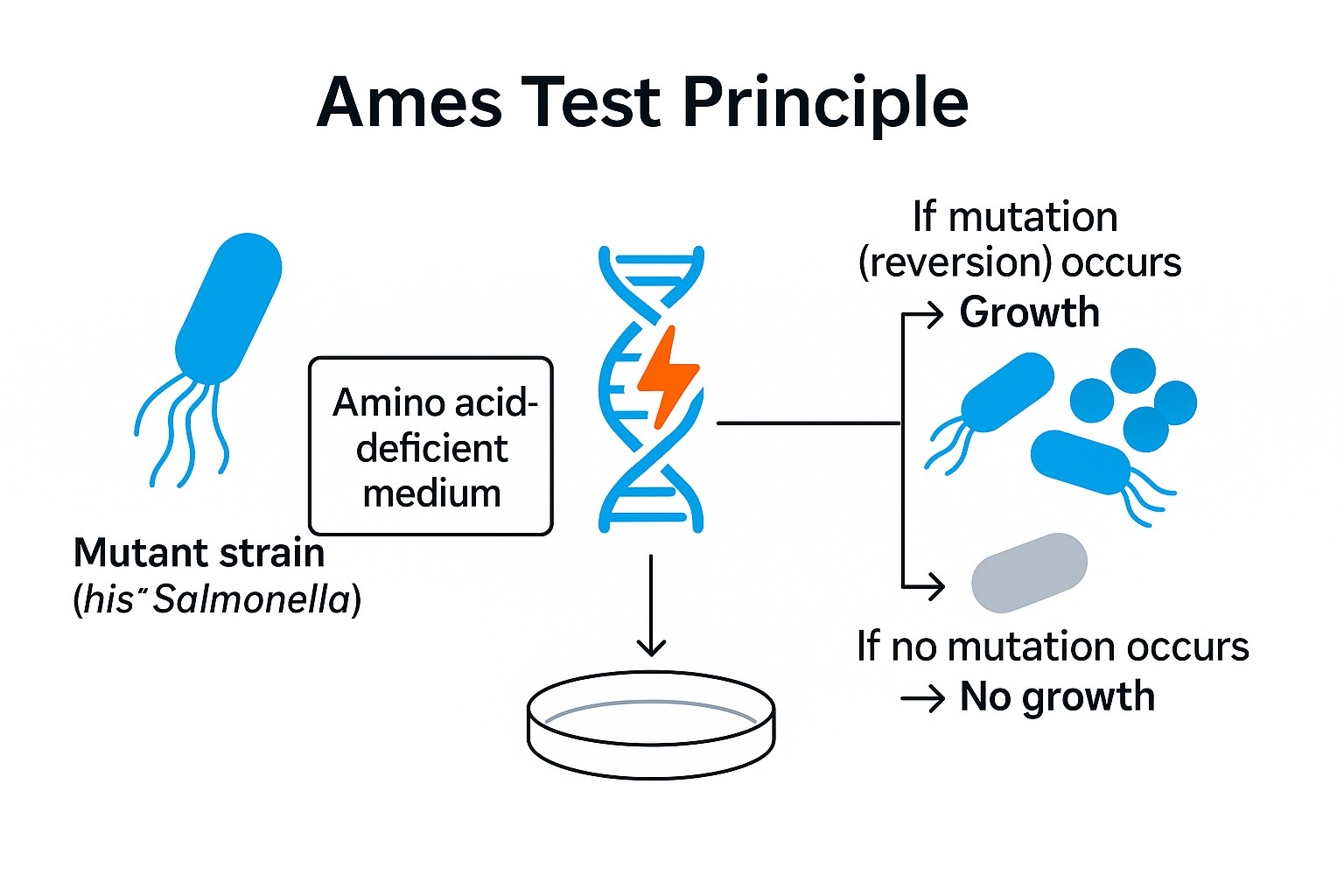
The Ames test, also known as the bacterial reverse mutation test, is the most widely used short-term assay for detecting mutagenicity. It evaluates whether a compound causes DNA damage leading to genetic mutations.
Ames test explained: This method relies on bacterial strains engineered with specific mutations that prevent them from synthesizing essential amino acids (histidine in Salmonella typhimurium, tryptophan in Escherichia coli). In the absence of mutagenic compounds, these strains cannot grow on selective media. However, if the test compound induces a reverse mutation, the bacteria regain the ability to synthesize the amino acid, forming visible colonies.
Ames test purpose: By measuring the frequency of such reverse mutations, researchers can assess the mutagenic potential of chemicals, pharmaceuticals, agrochemicals, and environmental samples. Because mutagenicity often correlates with carcinogenicity, the Ames test is widely applied in toxicology and carcinogen screening.
Ames test microbiology: The assay is grounded in microbial genetics, using well-characterized bacterial systems to mimic the DNA damage pathways relevant to higher organisms.
Principle of the Ames Test
At its core, the Ames test evaluates whether a chemical can induce genetic mutations.
Test organisms:
- Salmonella typhimurium histidine-dependent strains.
- Escherichia coli tryptophan-dependent strains.
Mechanism:
- Mutant bacterial strains cannot synthesize histidine or tryptophan.
- When exposed to a mutagen, reverse mutations restore their biosynthetic capacity.
- These revertant colonies grow on selective agar plates lacking histidine or tryptophan.
- An increase in revertant colony count compared to controls indicates mutagenicity.
Metabolic activation:
Many compounds only become mutagenic after metabolic conversion. To simulate mammalian metabolism, an S9 fraction (rat or hamster liver enzymes induced with phenobarbital and β-naphthoflavone) is added. This step ensures the Ames test can detect both parent compounds and mutagenic metabolites.
This principle underpins the assay's utility in drug discovery, chemical safety testing, and carcinogen risk assessment.
Ames Tester Strains – How to Choose
Selecting the correct tester strains is critical to ensure comprehensive coverage of mutagenic mechanisms. Each strain is engineered with specific mutations and plasmids that make it sensitive to different classes of mutagens.
Common Ames Tester Strains and Features
| Strain | Mutation | Type | Target | rfa | uvr- | pKM101 | pAQ1 | Notes |
|---|---|---|---|---|---|---|---|---|
| Salmonella TA98 | hisD3052 | Frame-shift | GCGCGCGC | √ | B | √ | – | Detects frame-shift mutagens |
| TA100 | hisG46 | Base-pair substitution | GGG | √ | B | √ | – | Detects base-pair mutagens |
| TA1535 | hisG46 | Base-pair substitution | GGG | √ | B | – | – | Narrow-spectrum base-pair detection |
| TA1537 | hisC3076 | Frame-shift | +1 (near CCC) | √ | B | – | – | Frameshift sensitivity |
| TA97a | hisD6610 | Frame-shift | GGGGGG | – | B | √ | – | Detects specific frame-shift events |
| TA102 | hisG428 | Frame-shift | A:T | – | – | – | √ | Detects oxidative stress & cross-linking agents |
| E. coli WP2 uvrA [pKM101] | trpE65 | Base-pair substitution | A:T | A | √ | √ | – | Detects base-pair mutations and oxidative damage |
| E. coli UvrA | trpE65 | Base-pair substitution | A:T | A | √ | – | – | Sensitive to base-pair substitutions |
Key Genetic Markers:
rfa: mutation in LPS layer → increases permeability to chemicals.
uvr-: loss of DNA repair → enhances sensitivity to DNA damage.
pKM101: R-factor plasmid that boosts chemical- and UV-induced mutation rates, adds ampicillin resistance.
pAQ1: pBR322 derivative plasmid carrying arc gene and tetracycline resistance
How to Choose the Right Strains
For base-pair substitution detection:
Use TA100, TA1535, or E. coli WP2 uvrA.
These are essential when evaluating chemicals suspected of inducing point mutations.
For frame-shift mutation detection:
Use TA98, TA97a, or TA1537.
These are standard strains for screening industrial chemicals, agrochemicals, and pharmaceuticals.
For oxidative stress or DNA cross-linking agents:
Include TA102 or E. coli WP2 uvrA (with plasmids).
These strains are sensitive to oxidizing agents and broad-spectrum DNA damage.
For regulatory compliance (OECD 471):
A panel of at least five strains is required:
- TA98, TA100, TA1535, TA1537, and E. coli WP2 uvrA.
- Additional strains (e.g., TA102) can be added for enhanced coverage.
For N-nitrosamines or complex metabolites (Enhanced Ames):
Use the full regulatory panel with enhanced metabolic activation (rat + hamster S9 mix, pre-incubation method).
By tailoring strain selection to compound type and regulatory requirements, Creative Proteomics ensures maximum sensitivity and global compliance.
Our Ames Test Services
At Creative Proteomics, we provide a complete portfolio of Ames test formats to meet diverse research and regulatory needs. Each format is designed for different project stages—from early compound screening to OECD guideline-based regulatory submissions.
| Service Type | Description | Best For |
|---|---|---|
| Standard Ames Test (Plate Incorporation Method) | Classic, OECD 471-compliant test using Salmonella and E. coli strains. Colonies are counted on selective agar plates. | Regulatory submissions, GLP studies, pharmaceutical impurity testing |
| Micro Ames | Microplate-based miniaturized version. Requires smaller sample volumes and supports higher throughput. | Early-stage compound screening, limited sample availability |
| Ames MPF (Microplate Fluctuation Test) | Liquid-culture assay in 96-well plates using pH indicators. Detects mutagenicity without colony counting. | High-throughput chemical library screening, cost-effective early toxicology |
| Ames II | Uses six TA7001–TA7006 strains targeting all possible base-pair substitutions, plus TA98 for frameshift detection. Highly sensitive to point mutations. | Detailed point mutation profiling, compounds with suspected specific mutagenic pathways |
| Mini Ames | Scaled-down plate test with fewer replicates. Lower cost and faster turnaround. | Pilot projects, preliminary screening before full Ames testing |
Standard vs. Enhanced Ames Test
While the standard Ames test is suitable for most compounds, certain substances require an Enhanced Ames Test to avoid false negatives.
Enhanced Ames Test – for N-Nitrosamines and Complex Metabolites:
- Recommended for nitrosamine drug substance related impurities (NDSRIs).
- Uses higher metabolic activation capacity: 30% rat liver S9 + 30% hamster liver S9.
- Incorporates pre-incubation method for better sensitivity.
- Required by EMA/FDA for nitrosamine impurity assessments.
By offering both standard and enhanced Ames tests, Creative Proteomics supports clients in meeting global regulatory expectations.
Ames Test Procedure & Workflow
Our testing workflow is designed for precision, compliance, and reproducibility:
Strain Selection: Appropriate Salmonella and E. coli strains chosen per OECD 471 guidelines.
Metabolic Activation: ± S9 fraction to replicate mammalian metabolism.
Exposure: Test compound applied using plate incorporation or pre-incubation methods.
Incubation: Strains grown on selective agar or liquid culture.
Counting: Revertant colonies (or indicator changes) quantified.
Data Analysis: Comparison with negative and positive controls, statistical validation.
Reporting: Publication-ready results with expert interpretation.
This procedure ensures reliable detection of mutagens across multiple compound types.
Applications of Ames Test Service
The Ames test is used across multiple sectors:
Pharmaceuticals:
- Screening new chemical entities.
- Evaluating impurities and degradation products.
- IND and NDA enabling studies.
Biotechnology and CROs:
- Safety evaluation of research compounds.
- Rapid mutagenicity screening in discovery.
Food and Cosmetics:
- Safety testing of additives, preservatives, and cosmetic ingredients.
- Meeting global regulatory standards for consumer products.
Environmental Toxicology:
- Assessing mutagenicity in soil, water, air, and wastewater samples.
- Supporting ecological risk assessments.
Why Choose Creative Proteomics
Creative Proteomics provides a trusted platform for mutagenicity testing:
Proven Expertise: Decades of experience in genetic toxicology and microbiology.
Comprehensive Coverage: Full strain panel, multiple assay formats, and enhanced test options.
Regulatory Compliance: OECD 471 and ICH S2(R1)-aligned procedures.
High Data Quality: Reproducibility ensured through rigorous quality control.
Data Deliverables:
- Raw data and colony counts.
- Statistical analyses and significance testing.
- Publication-ready charts and expert interpretation.
Global Service Reach: Testing facilities and collaborative networks worldwide.
Frequently Asked Questions (FAQ)
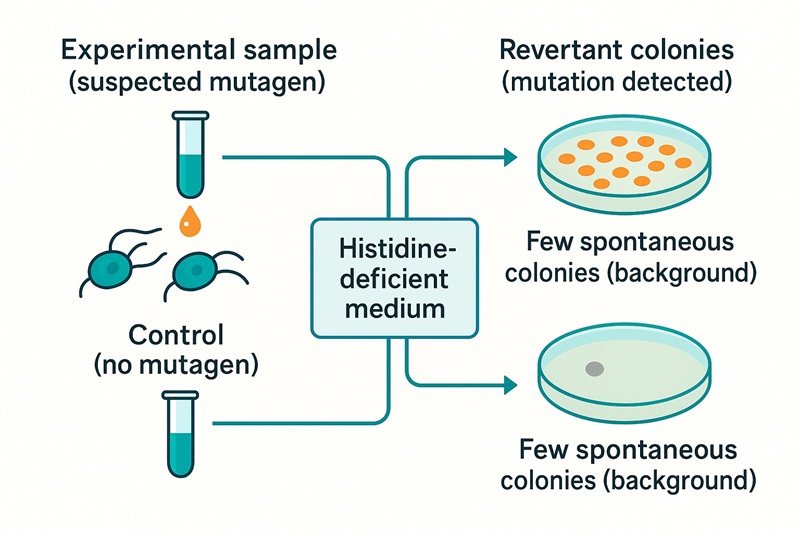
Do I need to include a negative control in an Ames test?
Yes. A negative or untreated control is essential to determine the background level of spontaneous revertant colonies. It is used alongside the positive control to validate the test results and confirm that the observed mutations are induced by the test compound and not by natural variability.
Why do some tests with S9 produce more or fewer colonies than those without S9?
This is normal and depends on the composition of the S9 metabolic activation system. Sometimes induced liver enzymes or residual inducers may reduce colony growth due to bacterial toxicity, while in other cases trace nutrients in the S9 mix can increase colony numbers. Both scenarios are expected and acceptable within regulatory guidelines.
How do I know if a test compound is cytotoxic to the bacteria?
Cytotoxicity is usually indicated when the number of revertant colonies decreases by more than 50% compared to the solvent control, when the background lawn of bacteria becomes thin or disappears, or when only pinpoint colonies appear at high doses. These signs suggest that bacterial growth is suppressed rather than that mutations are reduced.
Why is the Ames test performed with and without metabolic activation?
Some compounds are not mutagenic in their native form but become mutagenic after metabolic conversion in the body. The S9 fraction, which contains mammalian liver enzymes, allows the Ames test to detect these metabolites. Running the test both with and without S9 ensures that both the parent compound and its metabolic by-products are assessed.
How is the highest test dose determined in an Ames test?
The maximum dose is determined by both the solubility of the compound and its cytotoxicity to the bacterial strains. Pre-experiments are typically performed to evaluate solubility and toxicity, and the highest dose is set at the concentration where bacterial survival is still sufficient to evaluate mutation events.
Can low concentrations of a compound showing no effect mean that it has no genetic toxicity?
Not necessarily. A negative result at low concentrations does not prove the compound is non-mutagenic. Confirmation usually requires testing across multiple doses, adjusting experimental conditions, and in some cases combining the Ames test with other genotoxicity assays such as chromosomal aberration or micronucleus tests to reduce the risk of false negatives.
What solvents or vehicles can be used in an Ames test?
The solvent must not react with the test compound, must be non-toxic to the bacteria, and must not be mutagenic itself. Distilled water is preferred for water-soluble compounds. For water-insoluble or unstable compounds, organic solvents such as DMSO may be used. Regulatory guidelines limit the volume of organic solvent per plate to ensure accuracy.
What sample groups are typically included in an Ames test?
A complete Ames test generally includes an untreated control, a solvent control, one or more positive controls, and multiple dose groups of the test compound. If the solvent for the test compound differs from that of the positive control, an additional control for that solvent may also be required.
How many samples can be tested with an Ames test kit?
The number depends on the kit format and the number of strains included. For example, a 5-strain test with controls and replicates can support testing across several dose levels for a single compound, while smaller kits or miniaturized formats may allow multiple compounds to be screened simultaneously at reduced depth.
Does a positive Ames test result mean a compound is carcinogenic to humans?
Not directly. A positive result indicates that the compound has mutagenic potential in bacteria, which correlates with carcinogenic risk, but further studies in mammalian cells and in vivo systems are required to determine whether the compound poses a carcinogenic hazard to humans.
Learn about other Q&A about proteomics technology.
References
- Zeiger E. The test that changed the world: The Ames test and the regulation of chemicals. Mutat Res Genet Toxicol Environ Mutagen. 2019 May;841:43-48. doi: 10.1016/j.mrgentox.2019.05.007. Epub 2019 May 15. PMID: 31138410.
- Vijay U, Gupta S, Mathur P, Suravajhala P, Bhatnagar P. Microbial Mutagenicity Assay: Ames Test. Bio Protoc. 2018 Mar 20;8(6):e2763. doi: 10.21769/BioProtoc.2763. PMID: 34179285; PMCID: PMC8203972.












