Phytoceramides Analysis Service
Measure phytoceramides with decision-grade accuracy. Creative Proteomics quantifies phytoceramides and related sphingolipids—class, chain length, unsaturation, and hydroxy status—across complex matrices (skin tape-strips, cells, plant oils, and cosmetic formulations).
- Isomer-aware profiling: Cer[NP], Cer[AP], Cer[EOS]/EOP with class-matched internal standards
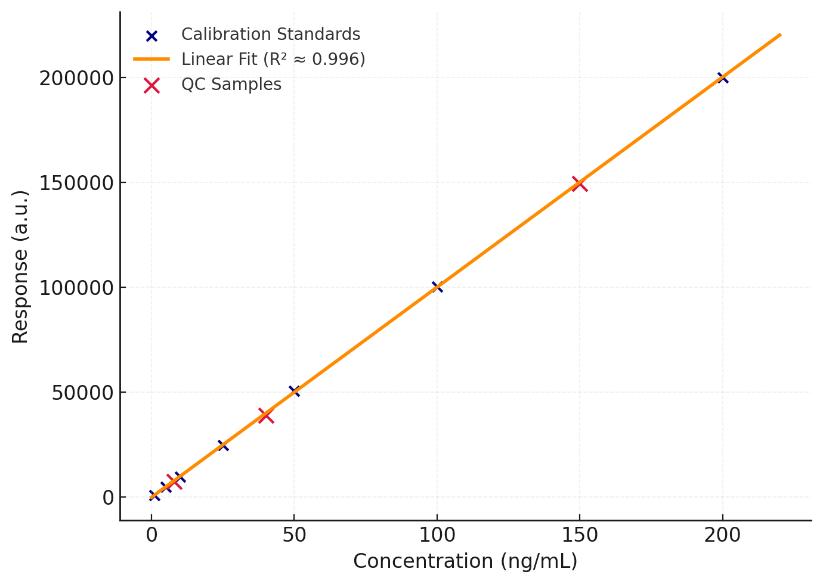
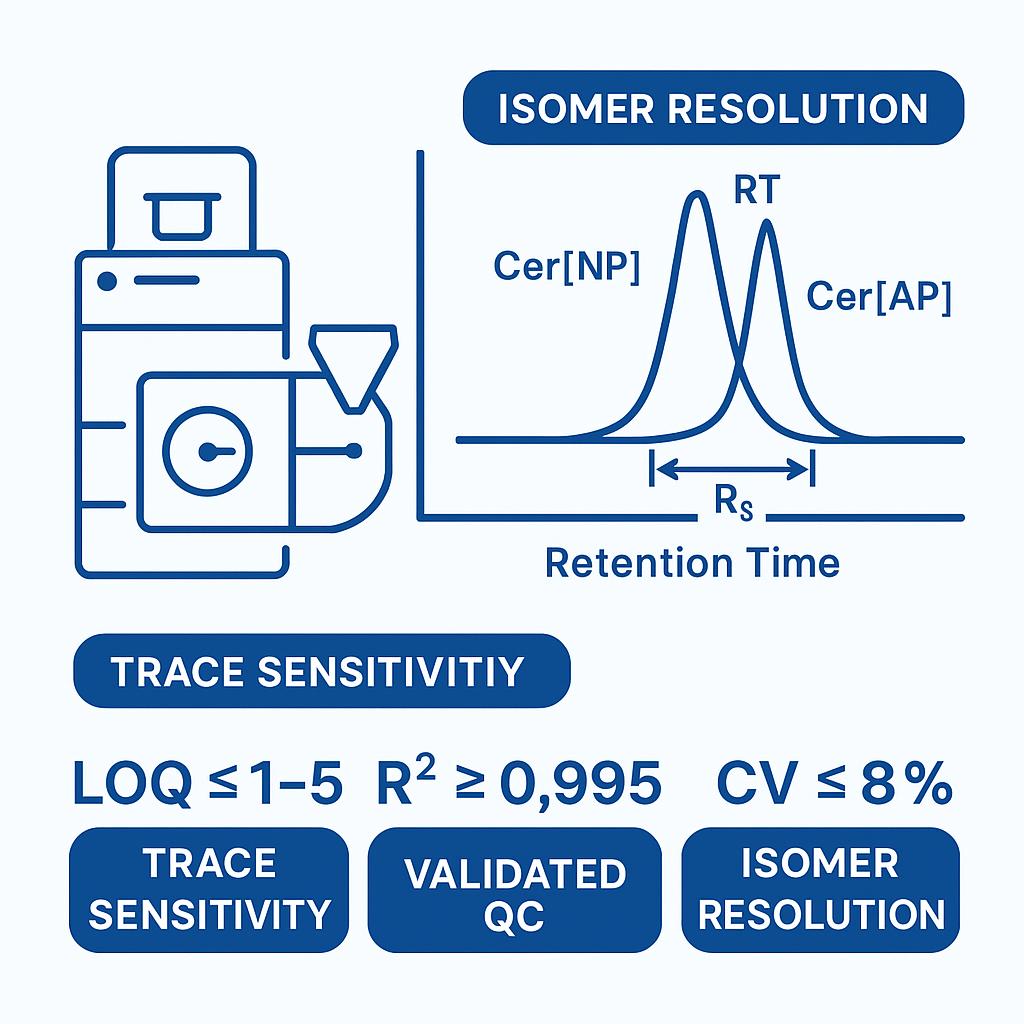
- Trace-level sensitivity: LOQ down to low-ng/mL (matrix-dependent) with R² ≥ 0.995
- Publication-ready outputs: Absolute/relative data, QC metrics, and annotated plots
Submit Your Request Now
×
What You Will Receive
- Raw MS files (.raw/.mzML) with full acquisition details
- Processed quantification tables with class-level and species-level data
- Calibration curves, QC reports, and precision/recovery metrics
- Annotated chromatograms and separation figures
- Data visualization: heatmaps, PCA, and class distribution plots
- What We Provide
- Advantages
- Technology Platform
- Sample Requirement
- Demo
- FAQs
What Are Phytoceramides?
Phytoceramides are a subclass of ceramides built on a phytosphingosine (t18:0) backbone, typically acylated with non-hydroxy (N), α-hydroxy (A), or esterified ω-hydroxy (E) fatty acids. They are key structural lipids in the stratum corneum and common components in plant-derived oils and cosmetic formulations. Variations in chain length (C16–C36), saturation, and hydroxylation directly influence barrier function, lipid phase behavior, and formulation stability. Due to their isomeric complexity and low abundance in some matrices, accurate profiling requires isomer-resolved LC–MS/MS with class-matched internal standards.
Phytoceramides Analysis Services Offered by Creative Proteomics
- Targeted Quantification (MRM/PRM): Absolute or relative quantification of Cer[NP], Cer[AP], and Cer[EOS]/EOP using class-specific internal standards. Applicable to skin, cell lysates, plasma, plant oils, and emulsions.
- High-Resolution Profiling (HRMS/ddMS²): Broad-spectrum detection and structural confirmation of phytoceramides and related sphingolipids. Ideal for discovery or low-abundance species.
- Isomer-Resolved Separation: HILIC-based LC–MS/MS resolves Cer[NP] vs Cer[AP] and identifies EOS-type species. Enables accurate class-level interpretation.
- Phytosphingosine & Phosphate Analysis: Quantification of t18:0 and t18:0-P for assessing biosynthetic and signaling pools.
- Stable Isotope Tracing (Optional)[¹³C]-labeled substrate tracing into phytoceramides for pathway flux analysis in cell models.
Analyte Coverage — Phytoceramides & Related Targets
| Category | Representative Analytes | Notes |
|---|---|---|
| Long-Chain Bases (LCBs) | Phytosphingosine (t18:0), t18:1, t18:0-P (phosphorylated), t18:1-P | Core sphingoid backbones in phytoceramides; phosphorylation states support signaling analysis |
| Cer[NP] (t18:0 + non-hydroxy fatty acid) | t18:0/16:0, 18:0, 20:0, 22:0, 23:0, 24:0, 24:1, 25:0, 26:0, 28:0, 30:0 | Most abundant phytoceramide class in skin and plant oils |
| Cer[AP] (t18:0 + α-hydroxy fatty acid) | t18:0/h16:0, h18:0, h20:0, h22:0, h23:0, h24:0, h24:1, h25:0, h26:0 | Often elevated in stratum corneum or used as formulation additives |
| Cer[AS] (d18:1 + α-hydroxy fatty acid) | d18:1/h16:0, h24:0, h24:1 | Can co-elute with phytoceramides; useful for class distinction |
| Cer[EOS]/[EOP] (ω-hydroxy fatty acid esterified with linoleic acid or similar) | t18:0/ω-OH-C28:0:18:2, C30:0:18:2, C32:0:18:2, C34:0:18:2, C36:0:18:2 | Barrier-relevant ceramides critical for lamellar lipid structure |
| Cer[EOH] / Cer[EOS-OH] | ω-hydroxy ceramides with terminal –OH or oxidized sidechains | Detected in some oxidatively stressed models or product degradation studies |
| Cer[NP]-d7, Cer[EOS]-d9, t18:0-d3 | Deuterated internal standards | Used for accurate quantification; isotope-labeled references for each class |
| Glycosylated Ceramides (GlcCer, GalCer) | GlcCer(t18:0/24:0), GlcCer(t18:0/26:0), GalCer(t18:0/24:1) | Key precursors and indicators of ceramide biosynthesis or topical delivery routes |
| Sphingoid Base Metabolites | t18:0 aldehydes, t18:0-1-dehydrogenase products | Oxidized/degradation forms indicating oxidative stress or pathway block |
| Other Plant Ceramides | t18:0/16:0, 18:2; d18:2/16:0 | Especially abundant in wheat germ, rice bran, konjac, and soy extracts |
| Related Lipid Classes (optional, if co-quantified) | HexCer(t18:0/24:0), SM(t18:0/16:0), dihydroceramides (d18:0/x:x) | Optional tracking to monitor pathway flux, especially in cell models or multi-lipid formulations |
| Oxidized Phytoceramides | t18:0/24:0-OH, t18:0/26:0:oxo, t18:0/30:0:OH | Detected under thermal/oxidative stress in formulations or aged biological samples |
Custom panels can be designed based on matrix type (e.g., skin, oil, serum, cream) and species origin (e.g., human, rice, konjac, synthetic), with tailored internal standard deployment and LOQ estimates.
Advantages of Our Phytoceramides Analysis Services
- Sensitivity: Method LOQs typically 1–5 ng/mL (or 5–25 ng/g) for major Cer[NP]/Cer[AP] species in common matrices; Cer[EOS]/EOP LOQs matrix-dependent due to chain length.
- Linearity: R² ≥ 0.995 across ≥ 3 orders of dynamic range with matrix-matched calibration.
- Precision: Intra-batch CV ≤ 8%; inter-batch CV ≤ 12% for monitor species under standard QC.
- Mass accuracy: High-resolution PRM at ≤ 3 ppm; MRM transitions verified by class-specific fragments.
- Isomer separation: Rs ≥ 1.2 between Cer[NP] and Cer[AP] on HILIC; reproducible RT windows (RSD ≤ 0.3%).
- Recovery & carryover: Spike-recovery 85–115%; carryover ≤ 0.2% with needle-wash and diversion logic.
- ID confidence: Retention-time match ±0.20 min, accurate mass/transition match, and MS² library score thresholds documented in the report.
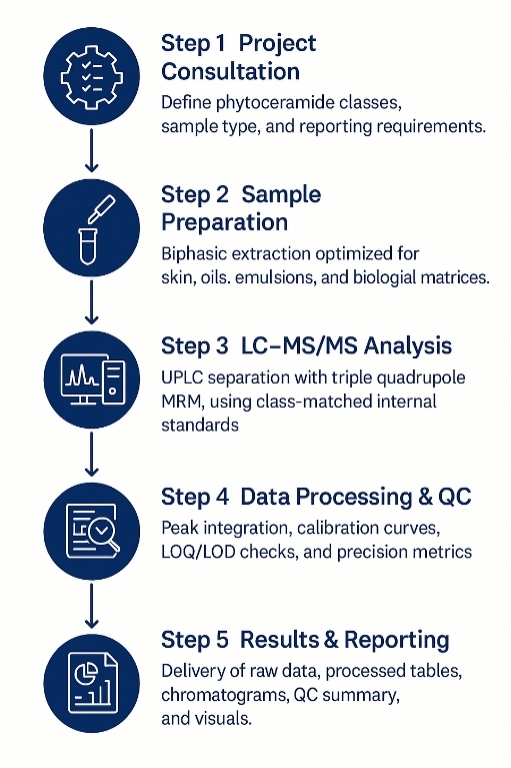
Workflow for Phytoceramides Analysis Service
1. Consult & Define Objectives — Matrix, target panel, quant type (absolute/relative), expected ranges.
2. Method Selection & Quotation — Choose APCI-MRM / HRAM / SFC and QC depth; finalize target list and calibration levels.
3. Sample Intake & Pre-QC — Receipt check, storage, and small-scale extraction test for recovery and matrix effects.
4. Extraction & ISTD Spiking — MTBE or hexane-based CE-preserving extraction with BHT; add deuterated CE ISTDs.
5. Chromatography & MS Acquisition — Gradient LC per above; scheduled MRM or HRAM PRM; pooled-QC every 10 samples.
6. Data Processing & QC Review — Calibration, RT/ion-ratio checks, blank/carryover review, drift correction if needed.
7. Reporting & Handover — Raw files, annotated tables, QC report, figures (TIC/XIC, calibration plots, heatmaps/PCA), and interpretation highlights.
Technology Platform for Phytoceramides Analysis Service
Chromatography
System: Waters ACQUITY UPLC I-Class or Thermo Vanquish
Column:
- HILIC (for isomer resolution): BEH Amide, 1.7 µm, 2.1 × 100 mm
- RP-C18 (for total class quant): BEH C18, 1.7 µm, 2.1 × 100 mm
Mobile Phase: ACN/IPA/H₂O mixtures with 10 mM ammonium formate or acetate, 0.1% formic acid
Flow Rate: 0.30–0.40 mL/min
Column Temp: 35–50 °C
Injection Volume: 2–5 µL (matrix-dependent)
Mass Spectrometry
Primary Instrument: Thermo TSQ Altis (Triple Quadrupole)
Ionization Mode: Positive ESI (Electrospray Ionization)
Scan Type: MRM (Multiple Reaction Monitoring)
LOQ Sensitivity: Typically 1–5 ng/mL for major species
Calibration: External, matrix-matched; with class-matched internal standards
Mass Accuracy: Within ±0.5 Da (low-res MRM); retention time window ±0.2 min
Precision: Intra-batch CV ≤ 8%; Inter-batch CV ≤ 12%

Waters ACQUITY UPLC System (Figure from Waters)

Thermo Fisher Q Exactive (Figure from Thermo)

TSQ Altis Triple Quadrupole MS (Figure from Thermo Scientific)
Sample Requirements for Phytoceramides Analysis Service
| Matrix | Minimum Amount | Container | Preparation Notes | Storage & Shipping |
|---|---|---|---|---|
| Skin tape-strips (stratum corneum) | ≥ 5 pooled strips per sample | Clean foil pouches or glass vials | Avoid lotions before sampling; label order of strips if needed | −80 °C; ship on dry ice; minimize freeze–thaw |
| Cell pellets | ≥ 1 × 10⁶ cells or equivalent protein | Low-bind tubes | Wash with ice-cold PBS; remove residual medium | −80 °C; dry ice |
| Tissue (animal/plant, research use) | 10–30 mg wet weight | Solvent-rinsed glass vials | Snap-freeze; record wet weight | −80 °C; dry ice |
| Plasma/Serum (research use) | ≥ 100 µL | Polypropylene cryovials | No additives containing glycerides/surfactants beyond standard anticoagulants | −80 °C; dry ice |
| Plant oils/extracts | ≥ 100 µL or ≥ 100 mg | Amber glass vials | Provide solvent system if pre-dissolved | Cold pack or dry ice as applicable |
| Creams/lotions/emulsions | ≥ 1 g | Wide-mouth amber glass | Provide formulation sheet (lipid phase %, surfactants) | Cold pack or dry ice as applicable |
| Reference standards (optional) | As available | Original vendor vials | Supply CoAs for any custom standards | Match matrix of main shipment |
If your matrix is not listed, we will specify equivalent mass/volume, container type, and handling to meet the same QC thresholds.
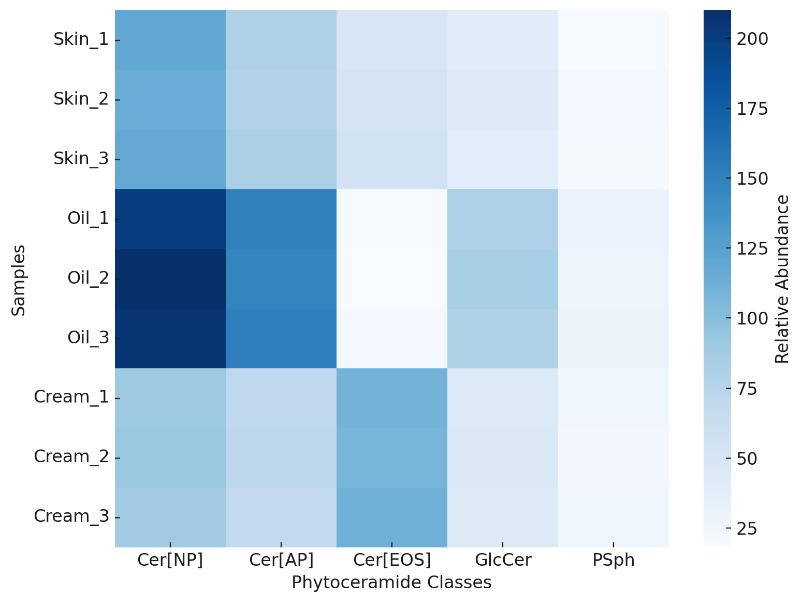
Demo Results
Applications for Phytoceramides Analysis
Skin Barrier Research
Quantify class-level and chain-length shifts in phytoceramides to assess barrier integrity and lipid organization.
Cosmetic Formulation Development
Evaluate phytoceramide stability, incorporation, and performance in creams, emulsions, and topical products.
Plant-Derived Lipid Characterization
Profile phytoceramide composition in natural oils, grains, and botanical extracts for ingredient sourcing.
Lipidomics & Metabolic Studies
Map phytoceramide subclasses as part of broader sphingolipid pathway and flux analyses.
Quality Control in Manufacturing
Implement targeted panels for batch consistency and raw material verification.
Stress & Stability Testing
Monitor oxidation, degradation, and class redistribution under storage or formulation stress conditions.
FAQ of Phytoceramides Analysis Service
What are the critical factors that influence data reliability in phytoceramide analysis?
Reliability depends on validated calibration strategies, class-specific internal standards, QC checkpoints, and reproducible separation that prevents misclassification of lipid subclasses.
How does LC–MS/MS provide defensible separation of phytoceramide isomers?
Optimized retention windows and selective MRM transitions allow clear distinction between Cer[NP], Cer[AP], and Cer[EOS], ensuring accurate isomer profiling.
Why are internal standards considered essential in phytoceramide quantification?
They correct for matrix effects and extraction variability, enabling robust absolute and relative quantification across diverse sample types.
How can phytoceramide profiling add value to cosmetic or formulation research?
It verifies phytoceramide stability and incorporation, supports formulation optimization, and provides quantitative benchmarks for batch consistency.
What measures are taken to minimize artifacts during phytoceramide analysis?
Antioxidant-protected solvents, low-temperature extraction, and validated handling protocols reduce oxidation and degradation artifacts.
How should phytoceramide results be interpreted across different sample sources?
Comparisons are best made using normalized class ratios, distribution profiles, and multivariate analysis across skin, botanical extracts, and emulsions.
How can phytoceramide analysis differentiate between endogenous skin lipids and exogenous formulation-derived ceramides?
By using isotope-labeled standards or matrix-matched calibration, endogenous pools can be distinguished from formulation inputs.
What are the typical challenges when analyzing phytoceramides in high-lipid or surfactant-rich matrices?
Co-extracted components may cause ion suppression; optimized biphasic extraction and matrix-matched standards maintain quantitation accuracy.
How does chain-length distribution of phytoceramides inform product performance or biological studies?
Chain length profiles influence barrier organization and formulation stability, offering functional insights into lipid behavior.
Can phytoceramide analysis be integrated with broader lipidomics workflows?
Yes, it can be combined with glycosylceramides, sphingomyelins, and related lipids to deliver pathway-level interpretation.
What quality metrics should be included in a phytoceramide analysis report?
Reports should include LOQ/LOD, linearity (R²), intra- and inter-batch CV, recovery rates, and mass accuracy as proof of analytical rigor.
How can phytoceramide data support non-clinical product development?
Validated quantitative results can underpin technical dossiers, raw material evaluation, and product positioning in R&D contexts.
Why is isomer-resolved profiling considered essential for barrier lipid research?
Cer[NP] and Cer[AP] differ in structural roles; separating them provides meaningful insights into barrier function and lipid organization.
Learn about other Q&A about proteomics technology.
Publications
Here are some of the lipidomics-related papers published by our clients:

- White matter lipid alterations during aging in mice. 2024.
- Characterization of Dnajc12 knockout mice, a model of hypodopaminergia. 2024.
- Evidence for phosphate-dependent control of sphingolipid metabolism. 2024.
- The olfactory receptor Olfr78 promotes differentiation through sphingolipid regulation. 2024.
- Glucosylceramide is essential for Heartland virus replication. 2023.
- Lipid droplet-associated lncRNA LIPTER preserves cardiac lipid homeostasis. 2023.
- Loss of G0/G1 switch gene 2 (G0S2) promotes deregulated sphingolipid metabolism. 2022.
![LC–MS/MS chromatogram of phytoceramide isomers Cer[NP] and Cer[AP] with baseline separation and resolution value.](/upload/image/pic-phytoceramides-quantitative-analysis-4.jpg)