- Service Details
- Demo Results
- Case Study
What Is Protein Glycosylation
Glycosylation, the attachment of sugar moieties to proteins, is a post-translational modification (PTM) that provides greater proteomic diversity than other PTMs. Glycosylation is critical for a wide range of biological processes, including the attachment of cell to the extracellular matrix and intracellular protein-ligand interactions. This PTM is characterized by various glycosidic linkages, including N-, O- and C-linked glycosylation, glypiation (GPI anchor attachment), and phosphoglycosylation, etc.
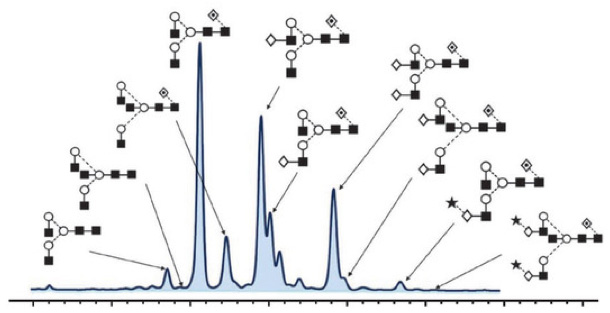
How to Analysis Glycosylation
The selection and application of glycosylation analysis techniques depend on the complexity of glycoproteins, their relevance to drug safety and efficacy, and the overall design of production process control strategies. Even though existing research indicates that glycosylation has no impact on biological activity, glycosylation control can serve as one of the measures to monitor production consistency. The choice of glycosylation analysis methods should be based on the level of information required to ensure the quality of glycoprotein products, including glycosylation distribution, glycosylation structure, and glycosylation site information.
The glycosylation analysis process typically involves a multi-step procedure, which may necessitate pre-processing steps (such as glycoprotein separation and purification) to eliminate interfering factors (e.g., excipients, salts). Completing the entire glycosylation analysis process involves four distinct complementary analytical methods: complete glycoprotein analysis, glycopeptide analysis, oligosaccharide analysis, and monosaccharide analysis.
In summary, advancements in new approaches and methods in the therapeutic monoclonal antibody and glycoprotein research field have continually enhanced and refined the quality of recombinant glycoprotein products.
The N-glycosylation and O-glycosylation analysis processes

Our Protein Glycosylation Analysis Service
Glycoproteins can be detected, purified and analyzed by different strategies.
With years of effort on developing Glycomics technologies, Creative Proteomics is willing to provide the following Glycomics services to assist the scientific research in the Glycomic area.
N-Glycan Profiling by MALDI TOF MS
N-Glycan Profiling by HPAEC-PAD
N-Glycan Profiling by HILIC-UHPLC
O-Glycan Profiling by MALDI TOF MS
O-Glycan Profiling by HPAEC-PAD
Glycomic Profiling Service by MALDI TOF MS
N-Glycan Linkage Analysis by HILIC-UHPLC
Others
Glycosphingolipid Glycans Analysis
N-glycan / O-glycan Profiling
Creative Proteomics offers N-glycan and O-glycan profiling through enzymatic treatment with PNGase F, which separates N-glycans from glycoproteins. The isolated glycans are then derivatized and labeled using the fluorescent reagent 2-aminobenzamide (2-AB). Subsequently, the labeled samples are subjected to UPLC separation, and relative quantification is achieved through area normalization based on the peak pattern observed on the fluorescence detector.

Sample Submission Requirements
Sample concentration ≥ 2.0 mg/mL;
Sample form: Liquid or formulation;
Storage: Frozen.
Protein Drug Glycosylation Site Analysis
Due to the lack of a template for the biosynthesis of glycans, different glycans can be attached to the same glycosylation site, leading to the phenomenon of microheterogeneity. The study of glycosylation sites is a crucial prerequisite for understanding the functionality of glycans. Precisely capturing and analyzing glycosylation sites forms the foundation for comprehending glycan functionality.
Creative Proteomics will tailor individualized enzymatic digestion protocols based on client requirements. Utilizing proteolytic enzymes, the tested samples will undergo enzymatic digestion. Glycosidase treatment will cleave the glycans linked to peptides. Leveraging a professional and efficient mass spectrometry platform, mature LC-MS/MS methods will separate and detect peptide mixtures. Data analysis software will be employed to analyze LC-MS and tandem mass spectra raw data, confirming changes in molecular weight after deglycosylation and determining peptide sequences with glycosylation modifications. This process aids in pinpointing glycosylation sites and provides comprehensive information on these sites.
Analysis Workflow:

Sample Submission Requirements:
Protein Sample: Quantity > 100 μg;
Sample Form: Liquid, lyophilized powder;
Storage: Low temperature (dry ice or ice packs).
Service Advantages
We employ rapid and efficient chemical derivatization labeling coupled with glycopeptide enrichment methods, enabling comprehensive and in-depth glycome profiling.
By implementing a comprehensive glycopeptide analysis strategy, we can simultaneously acquire information on glycosylation sites, glycan types, and paired qualitative and quantitative data.
Utilizing non-standard quantification methods, our approach requires minimal original sample volume and yields relatively low experimental errors.
Leveraging high-throughput, high-sensitivity mass spectrometry analysis techniques, our service offers a shortened turnaround time.
Creative Proteomics also provide the following bioinformatics services in Protein Post-translational Modification Analysis:
Functional annotation and enrichment analysis
Clustering analysis
Network analysis
Statistical analysis
Proteomic analysis of post-translational modifications
Please feel free to Contact Us to discuss your project. We hope you will find that we can meet your research needs.
 Glycan Profiling Peak Results Graph
Glycan Profiling Peak Results Graph

 TIC Chromatogram of Untreated N-Glycans (1) and N-Glycans After Enzymatic Cleavage (2)
TIC Chromatogram of Untreated N-Glycans (1) and N-Glycans After Enzymatic Cleavage (2)
| Sample name | Peptide | Modification information* | theoretical molecular weight (Da) | Measured Molecular Weight (Da) | |
|---|---|---|---|---|---|
| XXX | XXX | Glycan Untrimmed | G0F-GlcNAc | XXX | XXX |
| Glycan Cleavage | Deamidation (N149) | XXX | XXX | ||
Sample Glycosylation Modified Peptide Information

 Peptide Fragmentation (MS2) Spectra of Untreated N-Glycans (Top) and N-Glycans After Enzymatic Cleavage (Bottom) for Peptide Sequence (XXXXX)
Peptide Fragmentation (MS2) Spectra of Untreated N-Glycans (Top) and N-Glycans After Enzymatic Cleavage (Bottom) for Peptide Sequence (XXXXX)
Site-specific glycan analysis of the SARS-CoV-2 spike
Research Subject: SARS-CoV-2 Virus Spike Protein
Journal: Science
Impact Factor: 41.845
Research Background:
The emergence of the novel coronavirus SARS-CoV-2, the causative agent of COVID-19, poses a significant global threat to human health. Vaccine development has focused on targets that induce humoral immune responses, particularly the spike (S) glycoprotein, which mediates cell entry and membrane fusion. The SARS-CoV-2 S gene encodes 22 N-linked glycosylation sites, which may play a role in protein folding and immune evasion. This study employs site-specific mass spectrometry methods to reveal the glycan structures on the recombinant SARS-CoV-2 S immunogen, highlighting differences between SARS-CoV-2 S glycans and those of typical host glycan processing. These differences may impact virus pathobiology and vaccine design.
Sample Strategy: Purified recombinant SARS-CoV-2 Spike protein from 293F cells Technical
Strategy:
Mass spectrometry-based glycoproteomics
Results:
To address site-specific glycosylation of the SARS-CoV-2 S protein and visualize the distribution of glycan heterogeneity across the entire protein surface, researchers expressed and purified the recombinant SARS-CoV-2 Spike protein from 293F cells. An illustrative diagram of the SARS-CoV-2 S glycoprotein is shown in Figure 1A. Size exclusion chromatography was used to purify the recombinant SARS-CoV-2 S-containing supernatant, ensuring the analysis of natural trimeric protein only (Figure 1B). Negative stain electron microscopy verified the purified trimeric conformation (Figure 1C). Subsequently, the S protein was enzymatically digested using trypsin, chymotrypsin, and proteinase A, generating peptide fragments, each containing an N-glycan site. Mass spectrometry was employed to determine the glycan composition at each site.
 Figure 1. Expression and Verification of SARS-CoV-2 S Glycoprotein
Figure 1. Expression and Verification of SARS-CoV-2 S Glycoprotein
Using mass spectrometry, the 22 N-glycosylation sites on the S protein were determined (Figure 2), among which 18 glycosylation sites are conserved in the SARS virus S protein. Researchers observed that the glycan modifications at the S protein glycosylation sites could be categorized into three types: high mannose, hybrid, and complex glycan structures. The complex glycan type was found to be predominant, exhibiting substantial variation in the types and abundances of glycans at different glycosylation sites. A pie chart provides a quantitative summary of these glycans. Based on the abundance of high mannose glycans, sites were color-coded as green (80−100%), orange (30−79%), and pink (0−29%). Bar graphs represent the means of three biological replicates, with error bars indicating the standard error of the mean.
 Figure 2. Site-Specific N-Glycosylation of SARS-CoV-2 S Glycoprotein
Figure 2. Site-Specific N-Glycosylation of SARS-CoV-2 S Glycoprotein
By utilizing the electron microscopy structure of the S protein, the researchers mapped the glycosylation states onto the entire trimeric structure of the viral S protein, revealing how N-glycosylation is distributed across distinct regions of the SARS-CoV-2 S protein. The findings indicate that the extent of glycosylation on the SARS-CoV-2 S protein is comparatively lower and forms fewer "glycan shields" compared to other viral glycoproteins. This characteristic is favorable for the development of neutralizing antibodies. Additionally, the processing of complex glycans is a pivotal consideration in immunogen engineering.
 Figure 3. Structural-Based N-Linked Glycoprofiling of SARS-CoV-2 S
Figure 3. Structural-Based N-Linked Glycoprofiling of SARS-CoV-2 S
This study provides insight into the site-specific glycan characteristics of the naturally folded trimeric spike protein of SARS-CoV-2. As the development of glycoprotein-based candidate vaccines continues to grow, detailed glycan analysis offers a pathway for assessing immunogen integrity and becomes increasingly crucial for monitoring as production scales up for clinical use. Consequently, within the production of serological assay kits, glycan profiling analysis will also emerge as a vital indicator of antigen quality. Furthermore, as nucleotide-based vaccines emerge, understanding how these transmission mechanisms impact antigen processing and presentation becomes paramount.
References
- Watanabe Y, Allen JD, Wrapp D, et al. Site-specific glycan analysis of the SARS-CoV-2 spike. Science, 2020, 369:330-333.
- Ehret, Janike; Zimmermann, Martina; Eichhorn, Thomas; Zimmer, Aline (2018). Impact of cell culture media additives on IgG glycosylation produced in CHO cells. Biotechnology and Bioengineering







