What Is Anomeric Configuration
Carbohydrates usually exist in a linear or in a cyclic form, but given the stability, it is common that they are inclined to form rings. During cyclization, the carbonyl moiety that formed part of that linear structure is transformed into a new stereocenter. Cyclization causes the formation of two new diasteriomers. They are different in the position of the attachment of a certain group to the new stereocenter. The new stereocenter is referred to as the anomeric carbon. The anomeric center of a sugar is a stereocenter created from the intramolecular formation of an acetal (or ketal) of a sugar hydroxyl group and an aldehyde (or ketone) group. The two stereoisomers formed from the two possible stereochemistries at the anomeric center are called anomers. These two different stereoisomers (anomers) are designated α- and β-anomers. Anomers are capable of interconverting. There is the so-called mutarotation equilibrium between the α- and β-anomers of glucopyranose in aqueous solution.
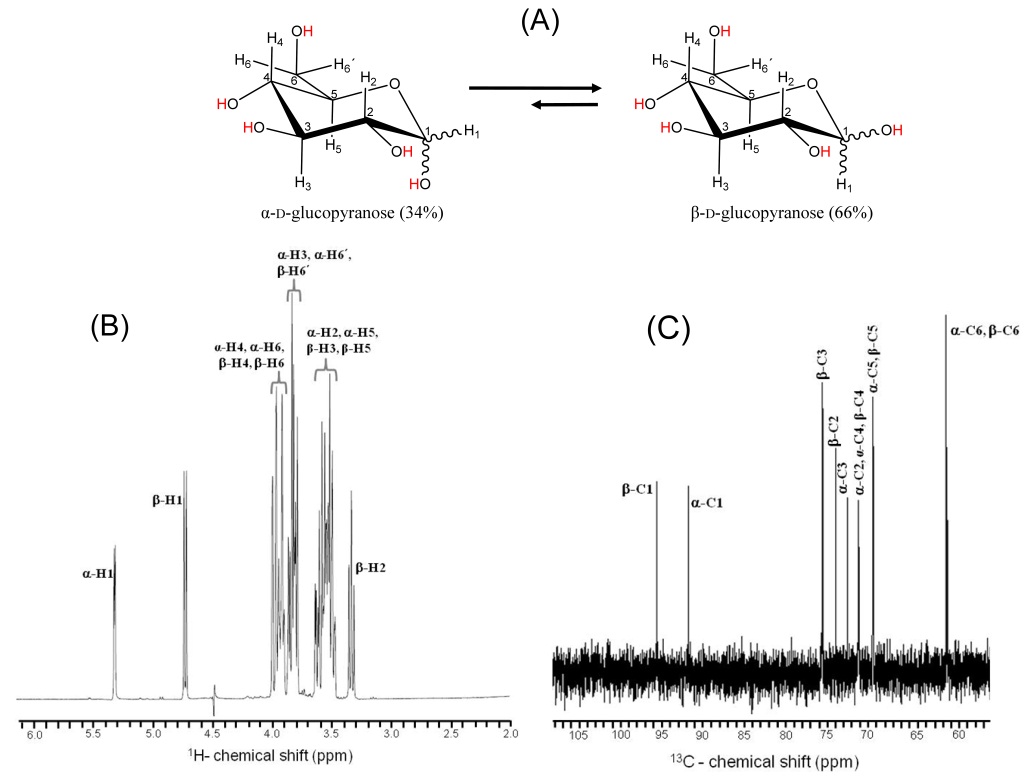 Figure 1. Structural representation of D-glucose and its anomeric configurations
Figure 1. Structural representation of D-glucose and its anomeric configurations
Polysaccharides with different anomeric configurations have different physical properties. They are different in structure, and thus have different stabilizing and destabilizing effects from each other. For example, the anomeric effect is one of the major contributors to the stability of a certain anomer. There is also the influence of anomeric configuration on mechanochemical degradation of polysaccharides.
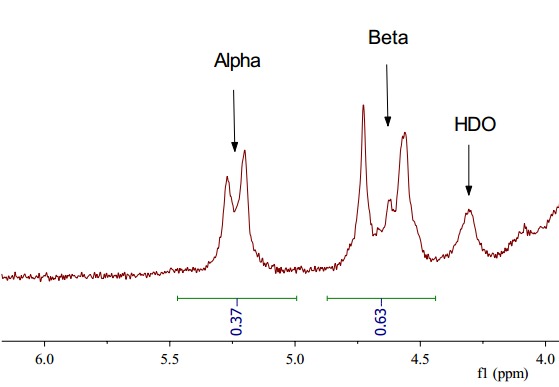 Figure 2. Example of 1H-NMR spectrum of α- and β–anomers
Figure 2. Example of 1H-NMR spectrum of α- and β–anomers
The one-dimensional Proton Nuclear Magnetic Resonance Spectroscopy (1H-NMR) has been a source of valuable structural information of polysaccharides such as the number and the configuration of anomeric protons. The signals arising due to anomeric protons usually appear in the range of 4.3~5.9 ppm, and also the protons of α-glycosides typically resonate 0.3-0.5 ppm downfield from those of the corresponding β–glycosides. In addition the α-anomeric proton resonates further down field (5.1 ppm) from the β–anomeric proton (4.5 ppm) making these two anomer distinguishable by 1H-NMR even at low field. The proton NMR spectrum also gives an idea about the constituent monosaccharides, based on chemical shifts and spin-spin coupling constants (JH-H). With knowledgeable experts and years of experience, Creative Proteomics offers accurate, reliable anomeric configuration analysis service.
Anomeric Configuration Profiling: Unveiling the Secrets of Sugar Molecules
At Creative Proteomics, with a deep-rooted passion for deciphering the language of sugars in biological systems, our team of glycoscience experts is dedicated to providing comprehensive analyses of anomeric configurations. Drawing from years of experience and armed with cutting-edge analytical tools, we are committed to uncovering the nuanced details of sugar molecules and their unique spatial arrangements. Whether your focus is on glycoproteins, carbohydrate-protein interactions, or glycan modifications, our services are tailored to elevate your glycoscience inquiries.
Services Tailored to Your Glycoscience Journey
Nuclear Magnetic Resonance (NMR) Spectroscopy: Immerse yourself in the world of sugar conformation with our advanced NMR techniques. Unveil the distinct spatial orientation of functional groups, shedding light on the structural nuances that define anomeric configurations.
High-Resolution Mass Spectrometry (MS): Our high-resolution MS analyses provide a window into the complexity of sugar molecules. Gain rapid and precise insights into anomeric configurations, particularly valuable for probing glycan structures and glycoconjugates.
Chiral Chromatography Mastery: Navigate the intricate landscape of anomeric configurations using our chiral chromatography techniques. Uncover the fine details of sugar molecule spatial arrangements, enabling comprehensive profiling and comparative studies.
Why Choose Us?
Glycoscience Pioneers: Our team comprises seasoned glycoscientists, deeply immersed in the intricacies of carbohydrate analysis, ensuring meticulous and reliable results.
Cutting-edge Instrumentation: Leveraging state-of-the-art instruments and methodologies, we provide data of the highest quality, advancing your glycoscience endeavors.
Customized Solutions: We recognize the unique aspects of each glycoscience project, tailoring our services to align with your specific objectives.
Timely Insights: We understand the urgency of your research, delivering comprehensive reports promptly to drive your glycoscience journey forward.
Unlock the Secrets of Anomeric Configurations
Unravel the hidden structural information encoded in sugar molecules with our Anomeric Configuration Analysis Services. Whether you're exploring fundamental biochemistry or seeking insights for pharmaceutical development, our expertise and advanced analytical techniques are here to empower your research. Contact us today to embark on a journey of discovery with anomeric configuration analysis.
How to place an order:





