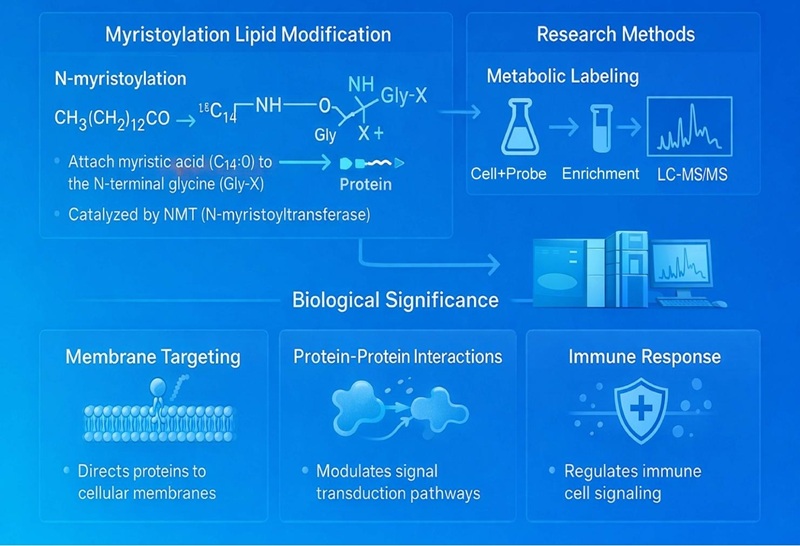
Myristoylation Analysis – LC-MS/MS Mapping of Protein N–Myristoylation Sites
Protein myristoylation is a lipid post-translational modification that covalently attaches a 14-carbon myristoyl group to the N-terminal glycine residue of target proteins, critically regulating membrane association, protein stability, intracellular trafficking, and signal transduction. Creative Proteomics offers comprehensive and high-precision myristoylation research services, including selective enrichment, site-specific identification, and quantitative profiling of myristoylated proteins by advanced mass spectrometry, enabling systematic investigation of myristoylation-regulated pathways and their biological and translational significance.
- Comprehensive profiling: Systematic identification and accurate quantification of myristoylated proteins, including precise, site-specific amino acid modifications.
- Advanced technological: Combination of metabolic labeling strategies with high-resolution LC-MS/MS to ensure high precision and in-depth coverage.
- Functional insights: Integrated bioinformatics for motif, pathway, and network interpretation.
- Research impact: Clarify the regulatory role of protein myristoylation in membrane targeting and signaling pathways, and to provide mechanism insights for disease biology and the discovery of therapeutic targets.
Submit Your Request Now
×
- Define
- What We Provide
- Technology Platform
- Workflow
- Advantages
- Applications
- Demo
- FAQs
- Case
- Publications
- Sample Requirement
What Is Myristoylation?
Myristoylation describes the addition of a C14:0 fatty acid linked to the free side chains of cysteines and lysines or N-termini glycines and cysteines of proteins[1]. N-myristoylation consists of the addition of the 14-carbon fatty acid, myristate, to the N-terminal glycine residue of a protein via a covalent amide bond. In rare cases, including those of Ras GTPases and TNF, myristic acid is attached to a lysine residue through an amide bound, a process named lysine myristoylation. N-myristoylation is catalyzed by N-myristoyltransferases (NMTs), which recognize specific N-terminal sequence motifs and utilize myristoyl-CoA as the acyl donor through an ordered enzymatic mechanism in which myristoyl-CoA binds first, followed by the substrate protein, enabling transfer of the myristoyl group to the exposed glycine[2]. Myristoylation can occur co-translationally after removal of the initiator methionine by methionine aminopeptidase or post-translationally when proteolytic cleavage generates a new N-terminal glycine. Once installed, myristoylation is irreversible and serves as a key determinant of membrane association, subcellular localization, protein–protein interactions, and signal transduction, thereby playing essential roles in processes such as innate immune signaling and host–pathogen interactions[3].
Myristoylation Service at Creative Proteomics
Owing to the critical role of protein myristoylation in regulating membrane targeting, signal transduction, and protein–protein interactions, protein myristoylation represents a fundamental regulatory mechanism and an emerging target in biomedical research. Therefore, systematic identification of myristoylated proteins and precise N-terminal modification sites within cells is essential for understanding their biological functions and disease relevance. We provide comprehensive myristoylation proteomics services, including selective enrichment strategies, high-resolution LC-MS/MS detection, site-specific identification, quantitative profiling, and advanced bioinformatics analysis. Our services enable researchers to globally characterize myristoylated proteins, delineate myristoylation-dependent signaling pathways, and explore their potential roles in disease mechanisms and therapeutic development.
Myristoylation Research Platforms

Thermo Fisher Easy-nLC 1000 and Thermo Fisher LTQ Obitrap ETD
(Figure from Thermo Scientific)
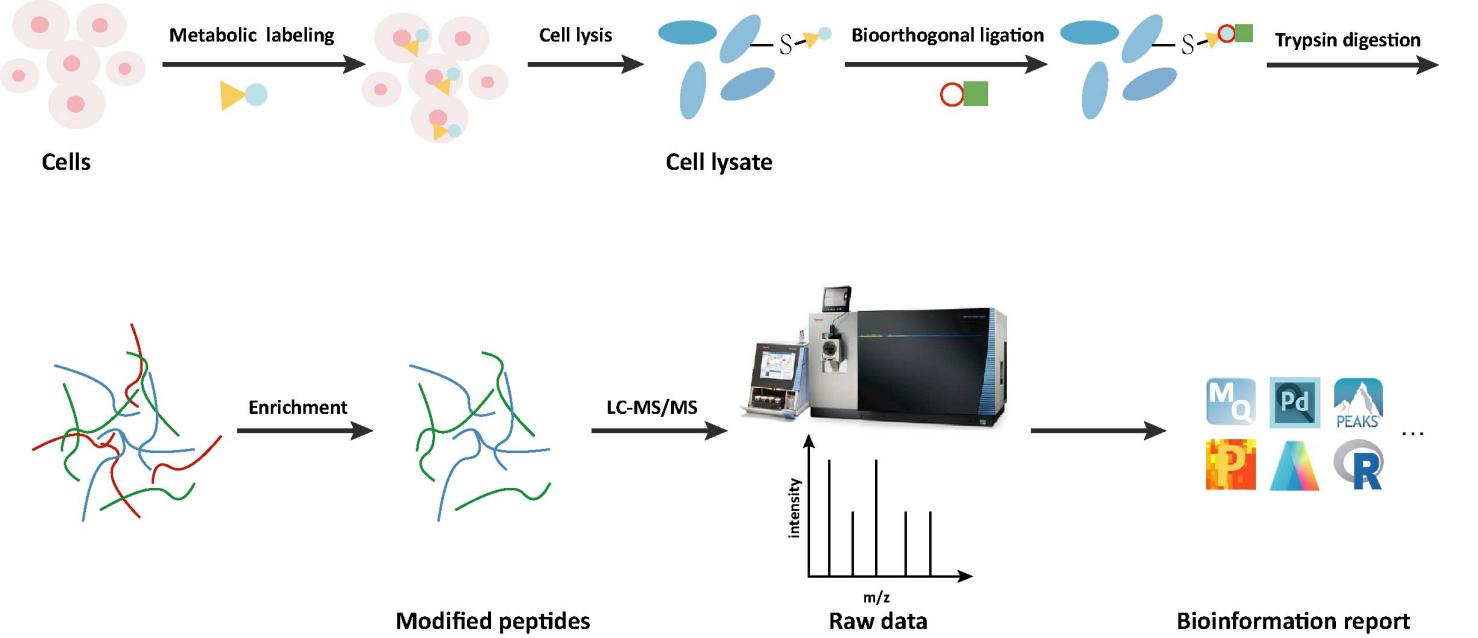
Workflow of MyristoylationAnalysis
Advantages Our Myristoylation Service
- Specific Enrichment Strategy: Utilize optimized metabolic labeling and chemical capture approaches to selectively enrich N-myristoylated proteins and peptides with high specificity.
- Site-Resolved Identification: Achieve confident, site-specific identification of myristoylation at N-terminal glycine residues using high-resolution LC–MS/MS.
- Quantitative Profiling Capability: Support comparative and quantitative analysis of myristoylation dynamics across different biological conditions or treatments.
- High Sensitivity and Reproducibility: Advanced sample preparation workflows and rigorous quality control ensure robust detection of low-abundance myristoylated proteins with excellent reproducibility.
- Biological Insight–Driven Analysis: Integrate bioinformatics and pathway analysis to link myristoylation events with protein function, signaling pathways, and disease relevance.
Applications of Myristoylation



The Potential of Drug Targets (NMT Inhibition)
N-myristoyltransferase (NMT) is regarded as an attractive drug target, as its inhibition can simultaneously affect multiple pathogenesis-related proteins that depend on myristoylation[3].
Tumour treatment
The abnormal activation of certain cancer-associated signaling pathways relies on myristoylated proteins. Targeting NMT is therefore considered a potential strategy for intervening in tumor signaling networks[2].
Anti-infection treatment strategy
NMT inhibitors have been explored for antiviral, antiparasitic and antifungal treatments, inhibiting the survival and spread of pathogens by blocking the mycolylation of key proteins[2][3].
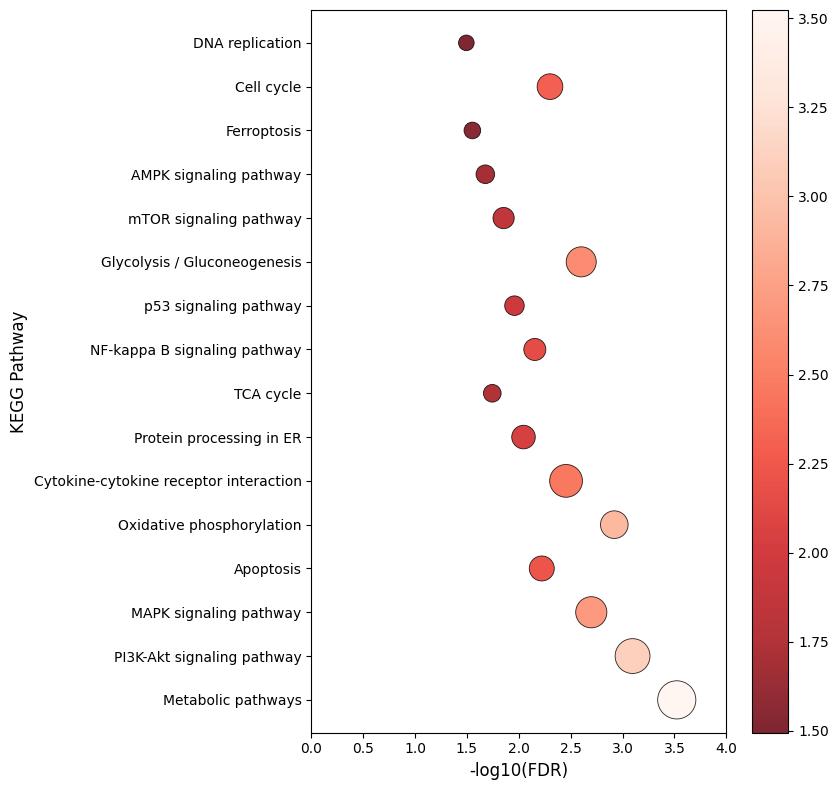
Demo Result of Myristoylation Service
FAQs of Myristoylation Service
Which sample types are accepted by the service?
We only accept samples of cell. We recommend that customers provide fresh or frozen samples, which should avoid repeated freezing and thawing, and we will be responsible for subsequent protein extraction and pretreatment.
Whether the sample needs pretreatment?
If you need this service, please contact us before cell culture. We will provide the probes for co-incubation with cells and the labeling methods.
What information is included in the final report?
The final report includes identified myristoylated proteins and sites, quantitative results, quality control metrics, and bioinformatics analyses such as functional annotation and pathway enrichment.
Learn about other Q&A.
Case Study

Publications
Here are some publications in Proteomics research from our clients:

- 3-D physiomimetic extracellular matrix hydrogels provide a supportive microenvironment for rodent and human islet culture. 2019. https://doi.org/10.1016/j.biomaterials.2018.08.057
- Confirmation of translatability and functionality certifies the dual endothelin1/VEGFsp receptor (DEspR) protein. 2016. https://doi.org/10.1186/s12867-016-0066-8
- Pressure overload induces ISG15 to facilitate adverse ventricular remodeling and promote heart failure. 2023. https://doi.org/10.1172/JCI161453
- Parkinson’s disease-associated LRRK2-G2019S mutant acts through regulation of SERCA activity to control ER stress in astrocytes. 2019. https://doi.org/10.1186/s40478-019-0716-4
- The cytoplasmic microtubule array in Neurospora crassa depends on microtubule-organizing centers at spindle pole bodies and microtubule+ end-depending pseudo-MTOCs at septa. 2022. https://doi.org/10.1016/j.fgb.2022.103729
Myristoylation Service Sample Requirements
| Sample | Sample Quantity | |
|---|---|---|
| Cell | suspension cell | > 3x108 |
| adherent cell | > 3x108 | |
References
- Giglione, C., & Meinnel, T. (2022). Mapping the myristoylome through a complete understanding of protein myristoylation biochemistry. Progress in lipid research, 85, 101139. https://doi.org/10.1016/j.plipres.2021.101139
- Yuan, M., Song, Z. H., Ying, M. D., Zhu, H., He, Q. J., Yang, B., & Cao, J. (2020). N-myristoylation: from cell biology to translational medicine. Acta pharmacologica Sinica, 41(8), 1005–1015. https://doi.org/10.1038/s41401-020-0388-4.
- Wang, B., Dai, T., Sun, W., Wei, Y., Ren, J., Zhang, L., Zhang, M., & Zhou, F. (2021). Protein N-myristoylation: functions and mechanisms in control of innate immunity. Cellular & molecular immunology, 18(4), 878–888. https://doi.org/10.1038/s41423-021-00663-2.