Title: Blood-based biomarkers and plasma Aβ assays in the differential diagnosis of Alzheimer's disease and behavioral-variant frontotemporal dementia
Journal: Alzheimer's Research & Therapy
Published: 2024
Background
The formation of neurotoxic amyloid beta (Ab) oligomers and plaques in specific brain regions is a key event in the pathogenesis of Alzheimer's disease (AD). The 42-amino acid isoform of Ab (Ab1-42) is thought to initiate plaque formation and AD progression. Various isoforms of Ab, such as Ab1-42, Ab1-40, and pGluAb3-42, have been detected in both sporadic AD (SAD) and familial AD (FAD) brains, but the relative importance of these isoforms in disease development is not fully understood. A study using immunoprecipitation and mass spectrometry analyzed Ab isoform patterns in the cerebellum, cortex, and hippocampus of AD subjects, including those with presenilin (M146V) or amyloid precursor protein (KM670/671NL) mutations, SAD patients, and non-demented controls. The results revealed that the dominant Ab isoforms in all brain regions were Ab1-42, pGluAb3-42, Ab4-42, and Ab1-40, with Ab1-42 and Ab4-42 being the primary isoforms in the hippocampus and cortex across all groups, including controls. There were no significant differences in Ab isoform patterns between FAD and SAD patients, suggesting similar amyloid pathology in both forms of AD.
Materials & Methods
IP-MS measurement of Aβ38, Aβ40 and Aβ42 in plasma samples (Ulm)
EDTA plasma samples, calibrators and QC samples (490μL each) were mixed with 15N-Aβ38, 15N-Aβ40 and 15N-Aβ42 (rPeptide, Watkinsville, GA, USA) as internal standards, with triethylammonium bicarbonate (TEAB, final 120mM) and Tween 20 (final 0.05%). Magnetic beads (0.5 mg per sample, Thermo 14302D) covalently coupled with 6E10 antibody (Biolegend, 2 μg/mg beads) were added to each sample and incubated on a rotator over night at 4 °C. Beads were washed three times with 500μL 50mM TEAB/0.1% n-Dodecyl-β-D-maltoside and eluted with 25μL 50mM glycine HCl (pH 2.5). The eluted Aβ peptides were digested with 10μL of a TrypN working solution (12.5ng/μL, Protifi, Fairport, NY, USA) for 2.5 h at 37 °C and stopped with 10μL of 0.5% TFA in acetonitrile and stored in the autosampler at 4 °C. A volume of 20μL was injected into a QTRAP6500 mass spectrometer (Sciex) coupled to an Eksigent MicroLC200 and Agilent 1260 pump. Peptides were loaded on an Acclaim PepMap100, C18 trap column (5 μm, 0.3×5 mm, Thermo) using mobile phase A: 0.05% TFA and mobile phase B: 90% acetonitrile, 0.1% ammonium hydroxide and a flow rate of 200μL/min. Separation of peptides was performed on a HALO Fused-Core C18, 100×0.5 mm analytical column (Eksigent, Framingham, MA, USA) at 60 °C and a gradient time of 9.85 min (5–35%B, total run time 17.5 min) with mobile A: 4% DMSO, 0.1% formic acid and mobile phase B: 4% DMSO, 96% acetonitrile, 0.1% formic acid. Peptides were infused into the QTRAP6500 mass spectrometer by electrospray ionization and measured in MRM mode using the following transitions: Aβ38 (aa28-38, 508.3→784.5 (b8+), 508.3→883.5 (b9+), 508.3→653.4 (b7+)); Aβ40 (aa28-40, 607.4→997.6 (b11+), 607.4→548.8 (b12++), 607.4→499.3 (b11++)); Aβ42 (aa28-42, 699.4→598.4 (b13++), 699.4→548.8 (b12++), 699.4→1096.7 (b12+)). Data were analysed using Skyline software v23.1 and for quantification, external calibration curves were generated using the light-to-heavy peak area ratios of calibrator samples and a quadratic function with 1/x² weighting. Calibrator samples (8-point calibration) were prepared in a surrogate matrix (3% bovine serum albumin in PBS) using synthetic Aβ38, Aβ40 and Aβ42 (Sigma) in the range of 1-100pg/mL (Aβ38, Aβ42) and 10-1000pg/mL (Aβ40). Plasma QC samples were included in all runs to monitor performance of measurements. The method was validated in terms of intraassay (1.2–10.5%) and interassay CV (3.4–7.4%), dilution stability (tested for 2- and 4-fold dilution, accuracy 89.9-110.7%), spike-in recovery (20pg/mL for Aβ38 and Aβ42, 200pg for Aβ40, recovery 93.1-100.4%) and stability at room temperature for 2h and up to 3 freeze-thaw-cycles (accuracy 80.2-108.1%). Intraassay CV of QC samples during measurement of patient samples was 1.2–10.5%.
Results
A strong correlation was observed between Aβ40 concentrations in patient samples, as determined by various assays (Fig. 1). The novel assay, referred to as "ULM," performed with IP-MS using ESI-MRM, demonstrated good correlation, with Spearman's rho values of 0.74 (P≤0.0001) when compared to the Shimadzu assay and 0.80 (P≤0.0001) when compared to the Simoa assay. For Aβ42 concentrations, a moderate to good correlation was seen, with Spearman's rho values of 0.61 (P≤0.0001) for the Shimadzu assay and 0.71 (P≤0.0001) for the Simoa platform. In contrast, the correlation for the Aβ42/40 ratio between the ULM assay and the Shimadzu assay was weak, with a Spearman's rho of 0.30 (P≤0.05), and a weak to moderate correlation of 0.46 (P≤0.001) with the Simoa platform's Aβ42/40 ratio. Comparatively, the correlations between the Shimadzu assay and the Simoa platform were modest but slightly lower, with Spearman's rho values of 0.68 (P≤0.0001) for Aβ40, 0.57 (P≤0.0001) for Aβ42, and 0.28 for the Aβ42/40 ratio. However, the latter correlation remained above the threshold of statistical significance (P>0.05).
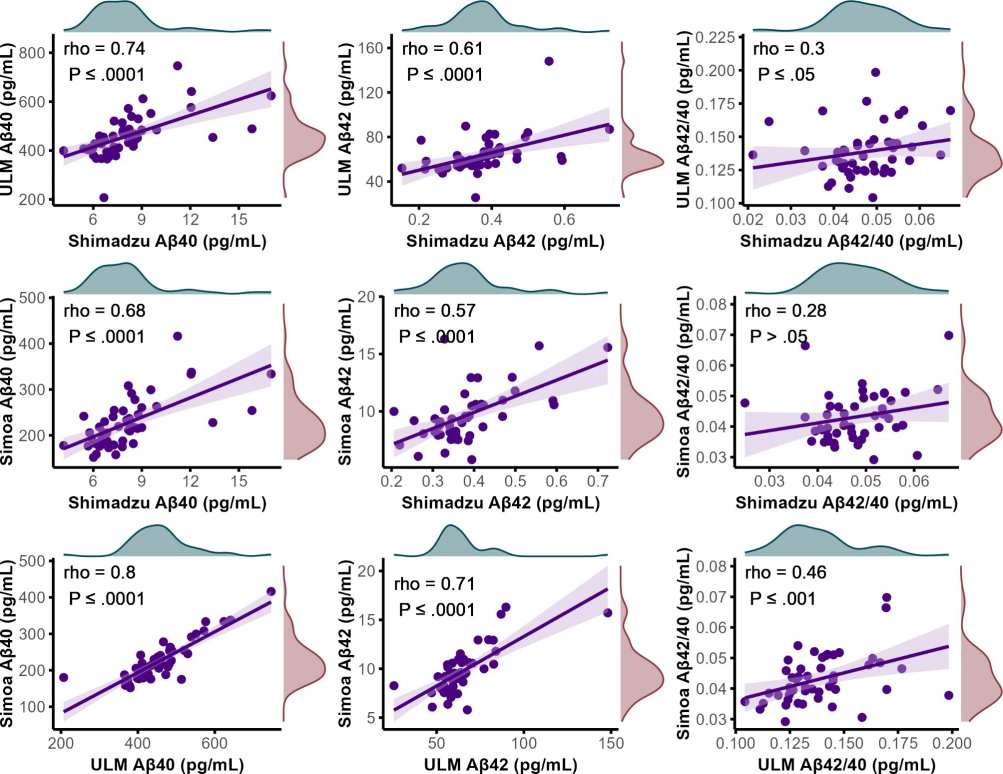
Figure 1. Scatter plots visualizing the correlations between plasma concentrations of Aβ40 and Aβ42, along with the Aβ42/Aβ40 ratio, across various as says.
Conclusion
To summarize, this study developed an IP-MS assay for Aβ42/40, which showed comparable accuracy to the Shimadzu composite score in distinguishing Alzheimer's disease (AD) from control groups. This new assay outperformed both the Shimadzu Aβ42/40 ratio and the Simoa Aβ42/40 assay. The results indicate that Aβ levels alone do not offer additional diagnostic value when differentiating AD from behavioral variant frontotemporal dementia (bvFTD). Importantly, the combination of pTau181 and GFAP proves to be a powerful tool for the blood-based differential diagnosis of AD and bvFTD.
Resource
- Mohaupt, P. et al. Blood-based biomarkers and plasma Aβ assays in the differential diagnosis of Alzheimer's disease and behavioral-variant frontotemporal dementia. Alz Res Therapy 16, 279 (2024). https://doi.org/10.1186/s13195-024-01647-w




