Title: BNT162b2 COVID-19 vaccination elicits the expansion of CD16+CD8+ T cells endowed with natural killer cell features
Journal: Journal of Allergy and Clinical Immunology
Published: 2025
Background
The Pfizer-BioNTech BNT162b2 vaccine, based on mRNA technology, demonstrated 95% efficacy against symptomatic COVID-19. While much of the focus has been on its adaptive immune response, including antibodies and antigen-specific T cells, the broader immune effects remain less understood. This study identified a significant increase in CD8+ T cells expressing FcγRIIIA (CD16), a receptor involved in antibody-dependent cellular cytotoxicity (ADCC), following BNT162b2 vaccination. CD16+ CD8+ T cells, which share characteristics with both T cells and NK cells, play a key role in the cytotoxic response. This finding suggests that the vaccine induces a hybrid T-cell subset with potent ADCC activity, contributing to the overall immune defense.
Materials and Methods
Culture Assay
NKp80+ and NKP80neg CD8+ T cells were sorted from healthy donors PBMCs using a fluorescence-activated cell sorting (FACSAria) instrument with the following strategy: CD3+CD8+CD45RA+CCR7negNKp80+/neg. Both subpopulations were cultured in complete medium supplemented with rhIL-15 (50ng/ml, Miltenyi) for 6 days in 96-well round-bottom plates. Cells were analysed for CD16 expression on day 2, 4 and 6 and for NKp30 expression on day 6, by flow cytometry. To determine the functional relevance of CD16 expression, cells were then tested in a 6h of degranulation assay following CD16 cross-linking alone or in combination with NKp80, as above described.
Flow Cytometry
Samples acquisition was performed on FACSCantoII or FACSymphony (BD Biosciences) flow cytometers and cell sorting was performed on FACSAria II cell sorter (BD Biosciences). Data were acquired by FACS Diva (BD Biosciences) and analysed by FlowJoVX (Tree Star Inc) software.
Results
NKp80+CD8+ T cells, which exhibit an effector memory re-expressing CD45RA (EMRA) phenotype, can upregulate the expression of both CD16 and NKp30 upon stimulation with IL-15. This was demonstrated by sorting NKp80+ and NKp80+CD8+ T cells from peripheral blood, followed by in vitro culture with IL-15 for six days. The NKp80+CD8+ T cells displayed a significant increase in CD16 and NKp30 expression, while the NKp80+CD8+ T cells did not show this upregulation. Furthermore, when stimulated with anti-NKp80 and anti-CD16 antibodies, the NKp80+CD8+ T cells exhibited a marked increase in both degranulation, as measured by CD107a expression, and IFN-γ production, compared to CD16 stimulation alone. This suggests that NKp80 and CD16 engagement together enhance the activation and functional response of CD8+ T cells with innate-like features, particularly after vaccination.
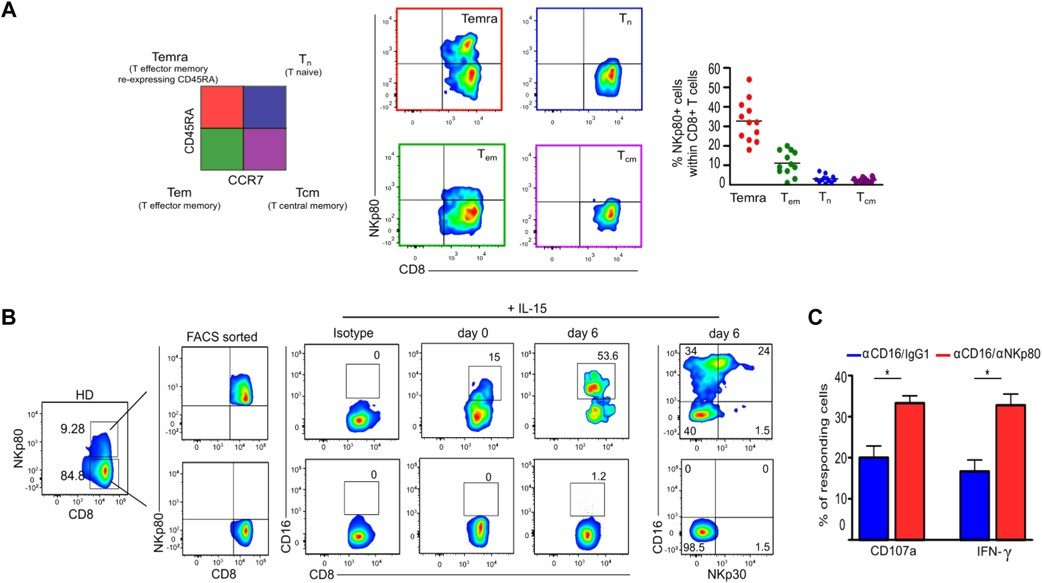
Figure 1. NKp80+CD8+ T cells acquire the expression of functional CD16 and NKp30 in the presence of IL-15
Conclusion
BNT162b2 COVID-19 vaccination generates a significant increase in effector cells with antibody-dependent cellular cytotoxicity (ADCC) capabilities, equipped with innate functions, which may enable them to combat a broader range of diseases, including cancer.
Resource
- De Pasquale.; et al. BNT162b2 COVID-19 vaccination elicits the expansion of CD16+CD8+ T cells endowed with natural killer cell features. The Journal of allergy and clinical immunology vol. 155,6 (2025): 1981-1992. doi:10.1016/j.jaci.2025.01.024




