Protein purity and homogeneity are key for protein crystallization and rigorous structural and functional studies. Impure samples will lead to inaccurate and imprecise results across experiments. In addition, Protein purity and homogeneity are two very important characteristics for further protein studies and biopharmaceutical applications. Purity of protein sample limits the application of protein products. Common contaminants of protein product include culturing medium and reagents from extraction and purification steps. In addition to impurity, protein heterogeneity may result from differential intra-cellular processing and the addition of co- and post-translational modifications. It is important to analyze the purity and homogeneity of your protein prior to using it for further processes.
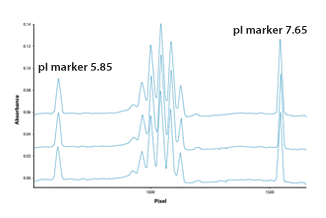
Figure 1. Protein purity and homogeneity characterization using pl markers.
Overview of Protein Purity and Homogeneity Characterization Service
At Creative Proteomics, we provide a complete list of protein purity and homogeneity characterization services to detect contaminants, protein variants, isomers, mismatched S-S link, truncated proteins, degraded proteins, protein modifications, protein aggregates, and protein precursors.
- Purity and integrity: SDS-PAGE, capillary electrophoresis, Zinc-reverse staining, glutaraldehyde-free modified silver staining, MALDI-TOF mass spectrometry
- Homogeneity assay: dynamic light scattering, UV-visible fluorescence spectroscopies, size-exclusion chromatography, static light scattering
Protein Purity Determination by SDS-PAGE
Protein purity evaluation is attainable through the utilization of SDS polyacrylamide gel electrophoresis (SDS-PAGE). Employing either SDS-PAGE electrophoresis or capillary electrophoresis (CE-SDS) affords the simplest, most cost-effective, and highly sensitive avenue for ascertaining the constituent count of proteins within a specimen, rendering it the prevailing technique. SDS gel electrophoresis, in conjunction with techniques harnessing electrophoresis for molecular weight and size determination, can be independently employed to appraise the purity of samples. The choice of method hinges on the intrinsic characteristics of the investigated protein.
Protein SDS-PAGE Analysis Procedure
Gel Preparation: Gels of varying concentrations are prepared based on the size of the protein sample. Higher concentration gels are used for smaller protein molecular weights, while lower concentration gels are suitable for larger proteins. The required volumes of stacking and separating gels differ according to gel thickness.
Loading and Electrophoresis: After the gel has partially solidified, protein markers, target identification proteins, and standard proteins (such as BSA) are loaded into sample wells. The power supply is then activated to initiate gel electrophoresis.
Staining and Destaining: Bromophenol blue tracking dye is allowed to migrate to the end of the gel. After de-stacking gel removal, the separation gel is stained in Coomassie brilliant blue staining solution for 40 minutes. Subsequently, the gel is placed in destaining solution for one hour, with the destaining solution replaced 2-3 times.
Results: Protein size is determined based on the marker (or standard curve derived from relative migration rates of protein standard markers), and protein purity is assessed by the presence or absence of impurity bands. A standard curve is constructed based on the grayscale of BSA standard bands, enabling the calculation of the corresponding concentration of the target protein.
Technical Features
The technique is highly mature, and the analysis results are accurate and reliable.

Protein Purity and Homogeneity Determination by HPLC
For analyzing protein purity, the simplest method is still SDS-PAGE. SDS-PAGE provides high resolution in electrophoretic separation. Even a 1 KD difference between two proteins can be discerned on an SDS-PAGE gel. However, due to the relatively low sensitivity of protein staining with Coomassie brilliant blue dye, impurities with protein content below 0.1 µg generally exhibit poor staining results and may not be effectively visualized on SDS-PAGE gels. Most purity analysis software can only perform rough calculations in such cases. Using HPLC analysis, protein purity is determined based on the separation of proteins according to their retention times, offering higher sensitivity and a more precise method for protein purity determination.
Size Exclusion Chromatography (SEC) separates proteins based on their hydrodynamic radius, which is directly proportional to molecular weight for most globular proteins. This method is often used to determine the content of monomers, high molecular weight aggregates, and low molecular weight fragments in monoclonal antibody products. Unlike SDS-PAGE, SEC does not disrupt the protein's three-dimensional structure and is suitable for separating and quantifying non-covalent aggregate forms. Due to its high throughput, compatibility with various detectors (UV detectors, light scattering, or fluorescence detectors), and ease of validation, SEC has become a primary technology in process development.
 Fast HPLC method
Fast HPLC method
We will select the most suitable method based on your requirements and sample characteristics.Our testing methods include, but are not limited to, the following:
Our Technology Platforms:
- Capillary Electrophoresis Sodium Dodecyl Sulfate (CE-SDS)
- Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
- Gel permeation chromatography (GPC)
- Size Exclusion Chromatography (SEC)
- Liquid Chromatography (LC)
- High Performance Liquid Chromatography (HPLC)
- Matrix Assisted Laser Desorption Ionization Mass Spectrometry (MALDI-MS)
- Electrospray Ionization Mass Spectrometry (ESI-MS)
- Circular Dichroism (CD)
- Nuclear Magnetic Resonance (NMR)
- Fluorescence Spectroscopy (Fluorometry)
- Dynamic Light Scattering and Static Light Scattering
Sample Requirements
We work with antibodies, antigens, enzymes, growth factors, DNA-binding proteins, blood proteins, membrane proteins, etc. We accept a range of samples, including:
- Native proteins from natural sources
- Fusion and tagged proteins from recombinant sources
- Antibodies of different isotypes
- Membrane proteins
Please submit at least 100 ug of purified protein at ≥1 mg/mL.
Advantages of Protein Purity and Homogeneity Characterization
- Advanced technical platforms. We are equipped with multiple technical platforms that allow to determine the protein purity and homogeneity accurately.
- Accuracy and reproducibility. We provide accurate and detailed results about the purity and homogeneity of your sample, which save you from wasting time on bad samples.
- A wide range of applications. Purified and homogenous proteins can be used in research and medical applications, such as the development and quality control of therapeutic proteins.
Creative Proteomics provides a wide spectrum of methods to characterize your samples cost-effectively, and delivers reliable and detailed data reports. As every project has different requirements, please contact our specialists to discuss your specific needs.
Reference
- Raynal B, Lenormand P, Baron B, et al. Quality assessment and optimization of purified protein samples: why and how?. Microbial cell factories, 2014, 13(1): 180






