As a pivotal post-translational modification in cellular signaling, protein phosphorylation regulates vital physiological processes including cellular growth, differentiation, metabolic activity, and responses to external stimuli. Researchers studying this modification typically employ two principal methodologies: targeted Protein Phosphorylation Assays and comprehensive phosphorylation omics. Each approach possesses distinct characteristics, making the selection of appropriate technology contingent upon specific research objectives and experimental settings. This paper provides a comprehensive overview of both techniques and offers guidance for selecting the optimal method under varying circumstances.
Technical Essence Comparison: Addressing Problems at Different Scales
Protein phosphorylation assay: quantitative analysis for specific phosphorylation sites
Targeted phosphorylation assays provide quantitative analysis of specific phosphorylation sites, typically focusing on individual or limited phosphosites. These methods employ antibodies or specialized reagents to measure phosphorylation levels via established techniques including enzyme-linked immunosorbent assay (ELISA), western blotting (WB), or immunoprecipitation. Key characteristics include:
- High Sensitivity: Detection of low-abundance phosphoproteins
- Quantitative Precision: Delivery of accurate phosphorylation metrics
- Target Specificity: Site-specific resolution enabled by antibody-based detection or molecular tags
- Experimental Applications:
- Ideal for investigations centered on predefined proteins or phosphorylation sites
- Validation of phosphorylation dynamics under controlled conditions (e.g., pathway activation post-treatment)
- Rapid, precise data generation for signal transduction studies with known targets
Phosphorylation Omics: comprehensively reveal the whole picture of phosphorylation modification
Phosphoproteomics enables comprehensive profiling of phosphorylation networks through systematic analysis of all phosphoproteins within biological samples. This approach employs high-throughput liquid chromatography-tandem mass spectrometry (LC-MS/MS), often coupled with data-independent acquisition (DIA) or MS/MS, to simultaneously quantify thousands of phosphorylation sites. Key characteristics include:
- Global Coverage: Identification of extensive phosphosites within single experiments
- High-Throughput Capacity: Scalable analysis for large sample cohorts
- Sample Versatility: Compatibility with complex matrices including cells, tissues, and clinical specimens
- Primary Applications:
- Exploration of phosphorylation landscapes in intricate biological systems (e.g., differential analysis between malignant and normal cells)
- Unbiased screening of phosphorylation alterations across physiological/pathological states with pathway-level interpretation
- Discovery of novel phosphosites and previously uncharacterized phosphoproteins
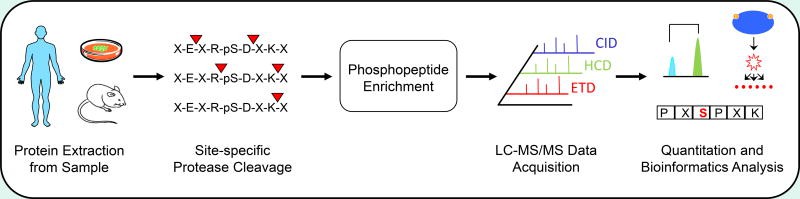 Typical bottom-up phosphoproteomics workflow (Arrington JV et al., 2017)
Typical bottom-up phosphoproteomics workflow (Arrington JV et al., 2017)
| Parameter | Phosphorylation Assay (Targeted Assays) | Phosphoproteomics |
|---|---|---|
| Detection Target | Specific protein(s)/site(s) (1-10) | System-wide sites (>1,000) |
| Core Technology | WB/ELISA/Imaging Mass Cytometry (IMC)/MRM | High-throughput LC-MS/MS + Enrichment Technology |
| Performance | High quantitative accuracy, Low throughput | Discovery-driven, Medium-to-high throughput |
| Dynamic Range | 10²-10³ | 10⁴-10⁵ (enrichment required) |
| Sample Consumption | Microgram level (WB) / Nanogram level (single-cell IMC) | Milligram level (tissue) / 10⁶ cell level |
| Turnaround Time | 1-3 days (single project) | 2-6 weeks (including data analysis) |
| Cost/Sample (USD) | $50 - $500 | $800 - $3,000 |
Select Service
Learn more
Decision Framework: Strategic Technology Selection for Phosphorylation Studies
Optimal choice of analytical tools is critical for experimental accuracy and reproducibility in phosphorylation research. This decision pathway provides guidance for target validation scenarios:
Scenario 1: Known Target Verification (Prioritize Targeted Assays)
Typical Applications:
- Drug mechanism studies (e.g., This study used targeted phosphorylation detection technology (including Western blotting, immunofluorescence and ChIP experiments) to verify the molecular mechanism by which cocaine promotes HIV transcription by regulating specific phosphorylation sites of key proteins (DNA-PK S2056, RNAP II CTD Ser5/Ser2, TRIM28 S824), providing a new target for the development of anti-HIV drugs targeting these phosphorylation sites (Sharma AL et al., 2024).)
- Diagnostic marker confirmation (e.g., In this study, a simple and reliable method for quantifying LRRK2 kinase pathway activity by measuring LRRK2-mediated Rab10 Thr73 phosphorylation levels in peripheral blood neutrophils was established through a specific phosphorylation detection technique based on the MJFF-pRab10 monoclonal antibody. This method was successfully applied to the analysis of clinical samples from patients with G2019S LRRK2-related and sporadic Parkinson's disease and healthy controls, confirming that peripheral blood neutrophils are a more ideal source of LRRK2 research samples than peripheral blood monocytes due to their high abundance, homogeneity, and high expression of LRRK2/Rab10 (Fan Y et al., 2018).)
When targets are predefined and phosphorylation status requires evaluation, site-specific detection becomes essential.
Technology Selection Pathway:
Experimental design begins with sample quantity assessment. For limited biological specimens (e.g., rare clinical biopsies or microdissected samples), single-cell resolution techniques are mandated, with Imaging Mass Cytometry (IMC) and phospho-flow cytometry providing spatial and population-level phosphorylation data respectively.
When adequate material is available (≥10⁶ cells or 1mg tissue), the requirement for absolute quantification determines subsequent methodology. Scenarios demanding precise stoichiometric measurements—particularly for low-abundance phospho-tyrosine (pY) sites—necessitate liquid chromatography-multiple reaction monitoring with MS³ fragmentation (LC-MRM/MS³), which enhances signal-to-noise ratios through orthogonal mass filtering.
Where relative quantification suffices (e.g., preliminary screenings or pathway validation), conventional Western blotting (WB) or enzyme-linked immunosorbent assays (ELISA) offer operationally simpler alternatives. This decision framework prioritizes analytical rigor while conserving precious samples, with antibody validation against knockout controls remaining essential for all immunoaffinity-based methods.
Implementation Guidelines:
- Small Sample Volumes: Utilize single-cell resolution methods (IMC, phospho-flow cytometry) for phosphorylation analysis when sample availability is constrained.
- Absolute Quantification Needs: Implement LC-MRM with MS³ for precise measurement of low-abundance targets (e.g., weak pY signals), where enhanced signal-to-noise ratios are critical.
- High-Throughput Screening: Employ WB or ELISA for rapid phosphorylation assessment when relative quantification suffices.
Critical Considerations:
- Antibody Validation: Prioritize specificity testing using knockout controls to eliminate cross-reactivity risks.
- Low-Abundance Targets: Apply MS³ methodology to improve detection specificity for faint phosphosignals (e.g., tyrosine phosphorylation).
Scenario 2: Investigating Unknown Mechanisms (Phosphoproteomics-Driven Approach)
When exploring novel biological mechanisms, researchers seek to uncover previously unrecognized phosphorylation-driven pathways or molecular disease subtypes. This complex analytical process necessitates comprehensive phosphoproteomic integration to ensure data reliability across all stages—from initial discovery to clinical validation.
Exemplar Applications:
- Drug Resistance Mechanisms: Identifying phosphorylation-mediated resistance pathways (eg., This study systematically analyzed the dynamic changes of insulin signaling networks in adipocytes and adipose tissues through phosphoproteomics technology. The study found that there was significant signaling network reprogramming in the state of insulin resistance, which was manifested by weakened insulin-responsive phosphorylation and the emergence of new insulin-regulated phosphorylation sites. The study identified multiple key dysregulated phosphorylation sites, revealing a signaling subnetwork including non-classical insulin regulatory factors such as MARK2/3, and found that the GSK3 signaling pathway was widely dysregulated. By establishing a kinase-substrate recognition analysis process, the specific changes in GSK3 substrates were confirmed (Fazakerley DJ et al., 2023).)
- Molecular Subtyping: Constructing phosphorylation-based classifications to discover subtype-specific biomarkers (eg., This study accurately divided high-grade serous ovarian cancer (HGSOC) into five molecular subtypes with significantly different prognoses through phosphorylation proteomics analysis, and its classification accuracy was better than traditional proteomic analysis. The study identified 29 abnormally activated kinases associated with poor prognosis, mainly involving PI3K/AKT/mTOR, cell cycle and MAP kinase pathways, and established a targeted treatment priority ranking system based on kinase activity characteristics. These findings provide an important basis for the precise classification and targeted treatment of HGSOC, highlighting the unique value of phosphorylation proteomics in precision medicine for tumors (Tong M et al., 2019).)
To learn more about the workflow, please refer to "Phosphoproteomics Workflow Explained: From Sample to Data".
Integrated Methodology:
- Stage 1: Discovery Phase
- Technology: Data-Dependent Acquisition (DDA) phosphoproteomics with TiO₂ enrichment
- Objective: Generate differential phosphosite lists (p<0.01, FC>2) potentially linked to novel mechanisms
- Stage 2: Targeted Verification
- Technology: Parallel Reaction Monitoring (PRM) or Data-Independent Acquisition (DIA)
- Objective: Quantitatively validate high-confidence candidates from discovery phase
- Stage 3: Clinical Translation
- Technology: Custom multiplex immunoassays (e.g., Luminex®/MSD)
- Objective: Verify biomarker clinical utility in patient-derived samples
Critical Diagnostic Development Pathway:
- Avoid direct translation from omics to diagnostics!
- A rigorous four-stage progression is essential:
- Discovery: Systematic phosphoproteomic identification of potential markers
- Verification: PRM-based target confirmation
- Standardization: MRM stability assessment and quality control
- Implementation: Development of clinically applicable detection assays
Decision Matrix for Phosphorylation Analysis Technology Selection
| Decision Dimension | Targeted Phosphorylation Assay | Phosphoproteomics | Critical Threshold Guidance |
|---|---|---|---|
| Research Objective | Known target validation (≤5 proteins/sites) | Novel mechanism discovery (>5 pathways/sites) | >5 targets requires omics screening |
| Sample Complexity | Homogeneous samples (cell lines, body fluids) | Heterogeneous tissues/microbiomes/clinical cohorts | Tissue samples mandate omics approach |
| Sample Input | Single-cell (IMC) or 10μg protein (WB) | ≥1mg tissue or 10⁶ cells (TiO₂ enrichment) | <100μg samples: avoid omics |
| Quantitation Precision | High (CV<15%, e.g. MRM) | Moderate (CV 15-25%, DDA mode) | IVD certification requires targeted assays |
| Cost/Time per Sample | $50-$500, 1-3 days | $800-$3,000, 2-6 weeks | <$10k budget: prefer targeted |
Sample Type-Specific Phosphorylation Analysis Guidelines
| Sample Type | Preferred Method | Critical Processing Technology | Quality Control Recommendations |
|---|---|---|---|
| Biopsy Tissues | Targeted Assay (MRM) | Laser Microdissection + pY Pre-enrichment | Limit freeze-thaw cycles to ≤3 |
| Serum/Plasma | Phospho-Antibody Array (MSD) | Phosphatase Inhibitor Tubes | Deplete High-Abundance Proteins (Albumin) |
| Tumor Microenvironment | Spatial Phosphoproteomics | Digital Spatial Profiling (DSP) + Multiplex IMC | Perform Total Protein Normalization |
| Bacteria/Fungi | Phosphoproteomics (DIA) | Bead-Beating Lysis + Ti⁴⁺-IMAC | Eliminate LPS Interference |
Comparison of Phosphorylation Enrichment Techniques
| Enrichment Technique | Principle | Target Sites | Recovery Rate | Common Interference Factors |
|---|---|---|---|---|
| TiO₂/MOAC | Metal oxide affinity chromatography | pS/pT | 70-85% | Acidic peptide competition |
| IMAC | Immobilized metal ion affinity chromatography | pY/pS/pT | 60-75% | Non-specific metal binding |
| Antibody Enrichment | Phospho-specific antibodies | Specific known sites | >90% | Cross-reactivity & non-specific binding |
To learn more about the mechanism and function of phosphorylation, please refer to "Understanding Protein Phosphorylation: Mechanisms and Biological Roles".
Future Fusion Trends: Next-Generation Solutions
1. Targeted Proteomics 2.0
Targeted proteomics will employ refined screening and quantitative methods to concentrate on disease-associated phosphorylation sites, moving beyond conventional large-scale genomics to prioritize critical signaling hubs. This innovation accelerates the understanding of molecular disease mechanisms. Key advancements include:
Core Signaling Hub Identification:
Phosphoproteomic analysis will be streamlined to 300 pivotal signaling nodes, enabling precise monitoring of essential biomarkers across diverse diseases or therapies. These hubs represent central regulatory points in key pathways.
AI-Guided Pathway Screening:
Artificial intelligence will pinpoint influential "pathway hub sites" (e.g., RAF-pS338 in MAPK pathways), identifying targets most impactful to disease progression and drug efficacy. This enhances drug target discovery and resistance mechanism analysis.
Applications:
- Precision Oncology: Customized cancer therapies leverage core phosphorylation hubs to rapidly assess tumor-specific drug responses (eg., This study successfully divided KMT2A rearranged AML into two distinct subtypes (MLLGA and MLLGB) by integrating multi-omics analysis such as phosphorylation proteomics. The phosphorylation proteomics characteristics revealed molecular characteristics such as enhanced DOT1L phosphorylation and increased CDK1 activity that are unique to the MLLGA subtype, and found that it has special sensitivity to 15 drugs such as IMPDH inhibitors. These findings not only clarify the heterogeneity mechanism of KMT2A rearranged AML, but more importantly, provide a direct basis for the development of precise treatment plans targeting the characteristic phosphorylation pattern of MLLGA (Casado P et al., 2023).)
- Metabolic Disease Diagnosis: AI-selected pathway hubs detect aberrant phosphorylation in conditions like diabetes or fatty liver for early intervention (eg.,By integrating multi-omics data from large databases such as TCGA and CPTAC, AI algorithms can accurately predict patient prognosis and identify therapeutic targets: 1) Boruta algorithm screens out phosphorylation markers related to liver cancer recurrence; 2) Convolutional neural network realizes the correlation analysis between renal cancer proteome and pathological images; 3) Joint learning model successfully builds a cross-cancer regulatory network. The study specially developed algorithms such as KSTAR, which can accurately predict HER2 targeted therapy response based on phosphorylation characteristics, and confirmed that dynamic phosphorylation analysis can optimize drug combination solutions (Varshney N et al., 2023).)
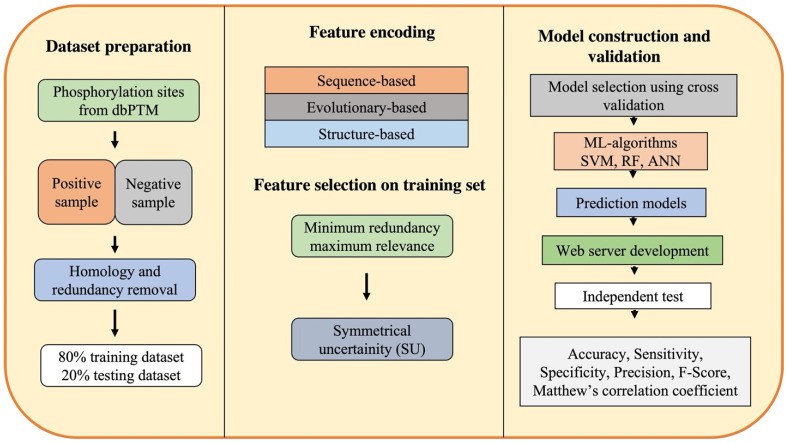 Workflow of machine learning-based computational approaches for phosphorylation site prediction (Varshney N et al., 2023)
Workflow of machine learning-based computational approaches for phosphorylation site prediction (Varshney N et al., 2023)
2. Integrated Real-Time Detection Platforms
Next-generation instant detection systems will revolutionize disease screening and clinical diagnostics. Combining microfluidics and nano-mass spectrometry, these platforms enable high-throughput biomarker analysis in under one hour.
Microfluidic System Integration:
Miniaturized devices consolidate the entire workflow—from cell lysis to phosphopeptide enrichment, nano-ESI, and mass spectrometry—simplifying operations and enhancing analytical precision.
Accelerated Detection:
Efficient phosphorylation profiling of 50 sites within 60 minutes expedites clinical decisions, facilitating faster personalized treatments.
Applications:
- Rapid Cancer Screening: Early tumor marker identification supports timely cancer detection and recurrence monitoring.
- Immune Response Tracking: Real-time immune status assessment during infections or post-vaccination enables dynamic therapy adjustments.
3. Digital Pathology Convergence
Integrating digital pathology with phosphoproteomics creates novel diagnostic frameworks. High-resolution spatial phosphorylation mapping and spatial transcriptomics (e.g., Visium HD) elucidate cellular interactions and pathway distributions in tissues.
Spatial Phosphoproteomics:
Coupling phosphorylation data with spatial pathology pinpoints signaling activities within tissue architectures, enriching cellular insights.
Phosphorylation Activity Mapping:
Patient-specific "phosphorylation activity landscapes" guide personalized therapeutic strategies based on disease states.
Applications:
- Tumor Microenvironment Analysis: Reveals regional phosphorylation variations in tumors, uncovering metastasis mechanisms and informing targeted drug design (eg., The spatially resolved phosphorylation proteomics (SRP) technology developed in this study accurately analyzed the endosomal signaling network during the FGFR2b receptor recycling process through biotinylation labeling and phosphopeptide enrichment. The study found that: 1) ULK1 S638 phosphorylation is specifically enriched in recycling endosomes, revealing that FGF10/FGFR2b regulates cell fate by inhibiting autophagy; 2) The core components of mTOR signaling are located in recycling endosomes, coordinating the balance between proliferation and autophagy; 3) 24.56% of FGF10-dependent phosphorylation is strictly dependent on receptor internalization. This technology provides a new perspective for understanding the spatiotemporal regulation of RTK signals (Watson J et al., 2022).)
- Neurodegenerative Research: Identifies brain-region-specific phosphorylation anomalies in Alzheimer's disease, enabling novel target discovery (eg., Phosphomic technology systematically analyzes the phosphorylation sites of TDP-43 protein (such as Ser409/Ser410) and its dynamic regulatory network, revealing the dual mechanism of action of this protein in neurodegenerative diseases such as ALS and FTD. Studies have found that phosphorylation mediated by specific kinases (such as c-Abl, CK1/2, TTBK1/2) can promote the pathological aggregation and mislocalization of TDP-43, while phosphatases (PP1/PP2A) have a protective effect. This technology not only identifies the phosphorylation features associated with disease progression, but also provides a molecular basis for the development of precision treatment strategies targeting kinase inhibitors (such as c-Abl inhibitors). In the future, it is necessary to further clarify its regulatory mechanism in combination with spatiotemporal dynamic analysis (Kellett EA et al., 2025).)
4. Single-Cell Phosphoproteomics Breakthrough
Technical Innovation:
- Resolution Enhancement: Integrated scPhos (10x Genomics) with timsTOF Pro 2 → >500 phosphosites/cell detection
- Spatial Mapping: Proposed Visium HD + Phospho-seq integration → tumor margin phosphoheterogeneity analysis
5. AI-Driven Predictive Frameworks
Computational Tools:
- Kinase-Substrate Prediction: DeepPhos deep learning tool → 82% validation accuracy for novel relationships
- Dynamic Signaling Modeling: Boolean network implementation → temporal phosphorylation prediction in EGF pathways
For more information on mass spectrometry and how to choose a platform, please refer to "Mass Spectrometry for Phosphoproteomics: Which Platform Is Best?".
People Also Ask
What is the most sensitive assay for phosphorylation?
LC-MS/MS and Enzyme-Linked Immunosorbent Assay (ELISA)
What is the difference between proteomics and phosphoproteomics?
Phosphoproteomics is a branch of proteomics that identifies, catalogs, and characterizes proteins containing a phosphate group as a posttranslational modification.
References
- Sharma AL, Tyagi P, Khumallambam M, Tyagi M. "Cocaine-Induced DNA-Dependent Protein Kinase Relieves RNAP II Pausing by Promoting TRIM28 Phosphorylation and RNAP II Hyperphosphorylation to Enhance HIV Transcription." Cells. 2024 Nov 23;13(23):1950. doi: 10.3390/cells13231950
- Fan Y, Howden AJM, Sarhan AR, Lis P, Ito G, Martinez TN, Brockmann K, Gasser T, Alessi DR, Sammler EM. "Interrogating Parkinson's disease LRRK2 kinase pathway activity by assessing Rab10 phosphorylation in human neutrophils." Biochem J. 2018 Jan 2;475(1):23-44. doi: 10.1042/BCJ20170803
- Fazakerley DJ, van Gerwen J, Cooke KC, Duan X, Needham EJ, Díaz-Vegas A, Madsen S, Norris DM, Shun-Shion AS, Krycer JR, Burchfield JG, Yang P, Wade MR, Brozinick JT, James DE, Humphrey SJ. "Phosphoproteomics reveals rewiring of the insulin signaling network and multi-nodal defects in insulin resistance." Nat Commun. 2023 Feb 18;14(1):923. doi: 10.1038/s41467-023-36549-2
- Tong M, Yu C, Zhan D, Zhang M, Zhen B, Zhu W, Wang Y, Wu C, He F, Qin J, Li T. "Molecular subtyping of cancer and nomination of kinase candidates for inhibition with phosphoproteomics: Reanalysis of CPTAC ovarian cancer." EBioMedicine. 2019 Feb;40:305-317. doi: 10.1016/j.ebiom.2018.12.039
- Casado P, Rio-Machin A, Miettinen JJ, Bewicke-Copley F, Rouault-Pierre K, Krizsan S, Parsons A, Rajeeve V, Miraki-Moud F, Taussig DC, Bödör C, Gribben J, Heckman C, Fitzgibbon J, Cutillas PR. "Integrative phosphoproteomics defines two biologically distinct groups of KMT2A rearranged acute myeloid leukaemia with different drug response phenotypes." Signal Transduct Target Ther. 2023 Feb 27;8(1):80. doi: 10.1038/s41392-022-01288-1
- Varshney N, Mishra AK. "Deep Learning in Phosphoproteomics: Methods and Application in Cancer Drug Discovery." Proteomes. 2023 May 2;11(2):16. doi: 10.3390/proteomes11020016
- Watson J, Ferguson HR, Brady RM, Ferguson J, Fullwood P, Mo H, Bexley KH, Knight D, Howell G, Schwartz JM, Smith MP, Francavilla C. "Spatially resolved phosphoproteomics reveals fibroblast growth factor receptor recycling-driven regulation of autophagy and survival." Nat Commun. 2022 Nov 3;13(1):6589. doi: 10.1038/s41467-022-34298-2
- Kellett EA, Bademosi AT, Walker AK. "Molecular mechanisms and consequences of TDP-43 phosphorylation in neurodegeneration." Mol Neurodegener. 2025 May 8;20(1):53. doi: 10.1186/s13024-025-00839-8
- Arrington JV, Hsu CC, Elder SG, Andy Tao W. "Recent advances in phosphoproteomics and application to neurological diseases." Analyst. 2017 Nov 20;142(23):4373-4387. doi: 10.1039/c7an00985b














