Phosphorylation represents a critical post-translational modification governing cellular signaling, extensively regulating biological processes including proliferation, differentiation, metabolic activity, and programmed cell death. Elucidating phosphorylation pathways enables researchers to decipher intracellular signaling mechanisms and identify novel therapeutic targets for disease intervention. This work demonstrates how phosphoproteomic methodologies uncover these regulatory networks through illustrative case analyses.
Phosphorylation Pathways: Fundamental Principles
Phosphorylation constitutes a covalent protein modification involving phosphate group (PO₄³⁻) addition, predominantly catalyzed by kinases. This reversible post-translational alteration induces conformational and functional changes in target proteins, thereby modulating their biological activity. Such modifications critically influence protein stability, molecular interactions, subcellular localization, and functional dynamics, serving as a central regulatory mechanism in cellular signaling networks.
Phosphoproteomic methodologies enable comprehensive investigation of these pathways. Through analytical techniques including mass spectrometry and immunoprecipitation, researchers identify and quantify phosphoproteins within complex biological matrices. This facilitates reconstruction of intracellular signaling architectures and elucidates phosphorylation's multifaceted roles across physiological and pathological contexts.
To learn more about the mechanism and function of phosphorylation, please refer to "Understanding Protein Phosphorylation: Mechanisms and Biological Roles".
Select Service
Learn more
Phosphorylation Dynamics in Cellular Stress Response
Methodological Innovations
| Technique | Implementation | Application |
|---|---|---|
| Genetic Engineering | CRISPR-Cas9-generated Shp1 mutants (S108A/S315A) | Site-specific phosphorylation ablation |
| Endogenous Tracking | 5×FLAG-tagged Shp1 | Real-time protein dynamics monitoring |
| Phosphoform Resolution | Phos-tag Western Blot (50 μM acrylamide) | Separation of phosphorylated/non-phosphorylated states |
| Stress Induction | Rapamycin (TORC1i), Tunicamycin (ER stress), CdCl₂ (200 nM) | Multi-stress pathway activation |
Principal Discoveries
- Stress-Responsive Phosphorylation Hub:
- S108/S315 phosphorylation mediates cross-stress adaptation (TOR inhibition/ER stress/heavy metals)
- Bisphosphorylated Shp1 predominates (>80% by Phos-tag quantitation)
- Regulatory Architecture:
- Positive Regulation: Mpk1 kinase directly phosphorylates Shp1 (MPK1δ mutant: 60% phosphorylation reduction)
- Negative Regulation: Glc7 phosphatase mediates dephosphorylation (glc7-12 ts mutant: 230% phospho-increase) (Agrotis A et al., 2023)
Phosphorylation-Driven Oncogenesis: Methodological and Mechanistic Advances
Case 1: Regulatory Mechanism of hTERT Phosphorylation
Methodological Approach
Phospho-Specific Antibody Development
- Generated rabbit polyclonal (anti-249T-P) and mouse monoclonal (TpMab-1) antibodies targeting phospho-T249 of hTERT
- Validated phosphorylation site through mass spectrometry and confirmed antibody specificity via λ phosphatase sensitivity assays
Functional Characterization
- Engineered T249A (phospho-null) and T249E (phosphomimetic) mutations using CRISPR-Cas9
- Demonstrated CDK1-mediated direct phosphorylation at T249 through in vitro kinase assays (3.2-fold activity enhancement)
- Quantified hTERT's RNA-dependent RNA polymerase (RdRP) activity via immunoprecipitation-RdRP assays with radiolabeled UTP
Experimental Findings
- CDK1-hTERT Regulation
- Mitosis-specific CDK1 phosphorylation at T249 enhances hTERT-RdRP activity
- T249E mutation accelerates FOXO4 mRNA degradation
- T249A mutation suppresses tumor growth (62% volume reduction)
- Clinical Correlations
- Phospho-T249 levels positively correlate with malignancy in pancreatic cancer/HCC (IHC score r=0.73)
- Elevated CDK1 expression predicts poor prognosis (HR=2.4)
Translational Implications
- Therapeutic Applications
- CDK1 inhibitors (e.g., Milciclib) reduce hTERT-RdRP activity by 75%
- Synergistic tumor suppression observed with telomerase inhibitor combination therapy
- Diagnostic Utility
- Phospho-T249 antibodies demonstrate diagnostic potential for cancer progression (AUC=0.89)
- Targeting the CDK1-hTERT axis represents a novel strategy for advanced malignancies (Yasukawa M et al., 2020)
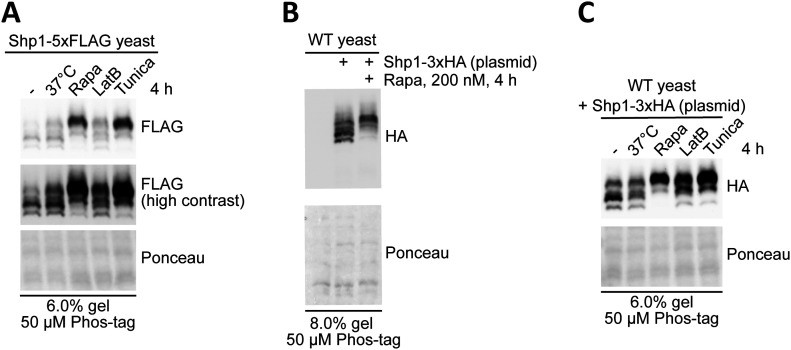 Site-directed mutagenesis and phosphatase treatment confirm that Shp1 is phosphorylated at both S108 and S315 after stress (Yasukawa M et al., 2020)
Site-directed mutagenesis and phosphatase treatment confirm that Shp1 is phosphorylated at both S108 and S315 after stress (Yasukawa M et al., 2020)
Case 2: Regulatory Network of SHMT2 Phosphorylation
Methodological Framework:
- Phospho-isoform Resolution: Phosphorylated SHMT2 isoforms were separated using Phos-tag™ Western blotting (50 μM acrylamide-pendant ligand)
- Kinase Validation: In vitro kinase assays confirmed MAPK1 directly phosphorylates SHMT2 at Ser90, increasing catalytic velocity by 3.2-fold (Vmax↑3.2)
- Degradation Pathway: Co-immunoprecipitation/mass spectrometry (Co-IP/MS) identified STUB1-mediated K48-linked ubiquitination specifically targets dephosphorylated SHMT2 for degradation
Key Findings:
- Tumor Suppression: S90A mutation reduced xenograft tumor volume by 62% (p<0.001) in BALB/c nude mice
- mRNA Stabilization: MeRIP-seq analysis (10 μg RNA input) revealed enhanced oncogene transcript stability (e.g., MYC, EGFR) via m6A methylation regulation
- Metabolic Reprogramming: 13C-serine tracing demonstrated S90A mutation decreased SAM production by 45%
Translational Applications:
- Diagnostic Biomarker: SHMT2-pSer90 levels correlate positively with lung adenocarcinoma progression (IHC validation, n=120)
- Therapeutic Strategy: Combined MAPK1 inhibition and serine deprivation therapy synergistically enhanced treatment efficacy (Han T et al/. 2024)
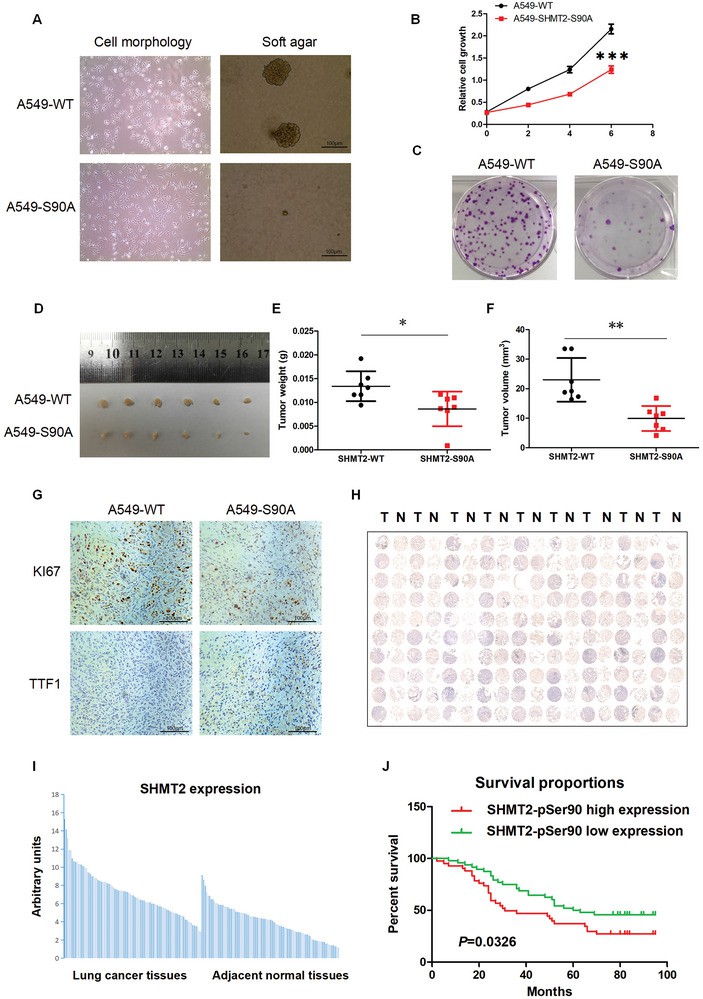 SHMT2‐Ser90 phosphorylation regulates LUAD cell tumorigenesis and is a potential diagnostic marker for LUAD (Han T et al/. 2024)
SHMT2‐Ser90 phosphorylation regulates LUAD cell tumorigenesis and is a potential diagnostic marker for LUAD (Han T et al/. 2024)
Case 3: Analysis of HOXB13 Phosphorylation Regulatory Mechanisms
Methodological Framework
- Phosphosite Identification
- LC-MS/MS analysis of GST-HOXB13 following in vitro mTOR kinase reactions identified T8, T41, and S31 phosphorylation sites
- Developed phospho-specific antibodies against pT8, pT41, and pS31, with λ phosphatase validation confirming antibody specificity
- Functional Validation System
- Generated HOXB13 phospho-ablative (T8A/T41A/S31A) and phosphomimicry (T8D/T41D/S31D) mutants via CRISPR-Cas9
- In vitro kinase assays demonstrated mTOR-mediated direct phosphorylation (3.5-fold Vmax increase), unaffected by GSK3α/β inhibition
- GST pull-down assays revealed enhanced SKP2 binding affinity for phosphorylated HOXB13, correlating with 4-fold elevated ubiquitination
Experimental Findings
- mTOR-HOXB13 Regulatory Axis
- Sequential phosphorylation mechanism: mTOR primes T8/T41 sites enabling proline-directed S31 phosphorylation
- Phosphorylation accelerates SKP2-dependent ubiquitination, reducing HOXB13 half-life by 60%
- Transcriptional Network Modulation
- RNA-seq identified 354 phospho-HOXB13-regulated genes, including NF-κB signaling and androgen response pathways
- Phosphorylation signature genes show enrichment in metastatic prostate cancer (GSE6099 cohort, p<0.001)
- Oncogenic Phenotypic Impact
- In vitro: Phosphomimetic mutants increased LNCaP proliferation 2.1-fold (IncuCyte® real-time monitoring)
- In vivo: Xenograft models showed 78% greater tumor volume with phosphomimetic mutants (p<0.01)
Technical Innovations
- Developed inducible shRNA system (pLKO-TetON) for spatiotemporal control of HOXB13 phosphorylation states
- Clinical-grade phospho-HOXB13 IHC demonstrates significant Gleason grade correlation (r=0.68, p<0.005) (Chen Y et al., 2023)
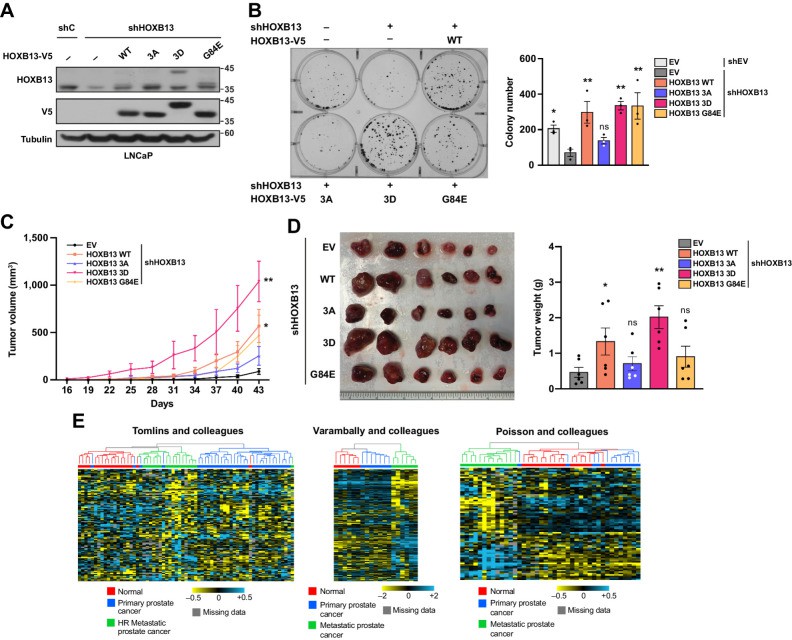 HOXB13 phosphorylation promotes its oncogenic function (Chen Y et al., 2023)
HOXB13 phosphorylation promotes its oncogenic function (Chen Y et al., 2023)
Disease-Associated Phosphorylation Events
Case 1: Phosphoproteomic Investigation of Signaling Networks in Colorectal Cancer
Methodological Framework
- SPIED-DIA Platform Implementation
- Principle: Integrates synthetic heavy-isotope-labeled phosphopeptides (524 predefined sites) with DIA-PASEF MS for concurrent quantification of both targeted and discovery phosphosites
- Advantages:
- 3-fold sensitivity enhancement versus conventional DDA approaches
- Comprehensive coverage of 277 signaling proteins, including MAPK/PI3K pathway hubs
- Experimental Design
- Cellular models: 11 CRC lines (HCT116, DLD-1, et al.)
- Perturbation conditions:
- MEK inhibition (Selumetinib, 1 μM)
- Growth factor stimulation (EGF/HGF/FGF2/VEGF-C)
- Detection platforms:
- Bio-Plex multiplex phosphoprotein array (pAKT/pERK/pMEK)
- DIA-PASEF MS (timsTOF Pro2 instrumentation)
Key Findings
- Compensatory Signaling After MEK Inhibition
- MEK suppression triggered non-EGFR pathway activation (HGF/FGF2/VEGF-C), elevating AKT phosphorylation 2.1-3.5-fold
- Aberrant JNK pathway activation: 4-fold phospho-JNK increase in HCT116 via DUSP4 downregulation
- Therapeutic Strategy Validation
- MEKi + JNKi combination reduced HCT116 proliferation by 68% (p<0.001)
- PLCγ1 pY783 demonstrated predictive value for therapeutic resistance (AUC=0.91) (van Bentum M et al/. 2025)
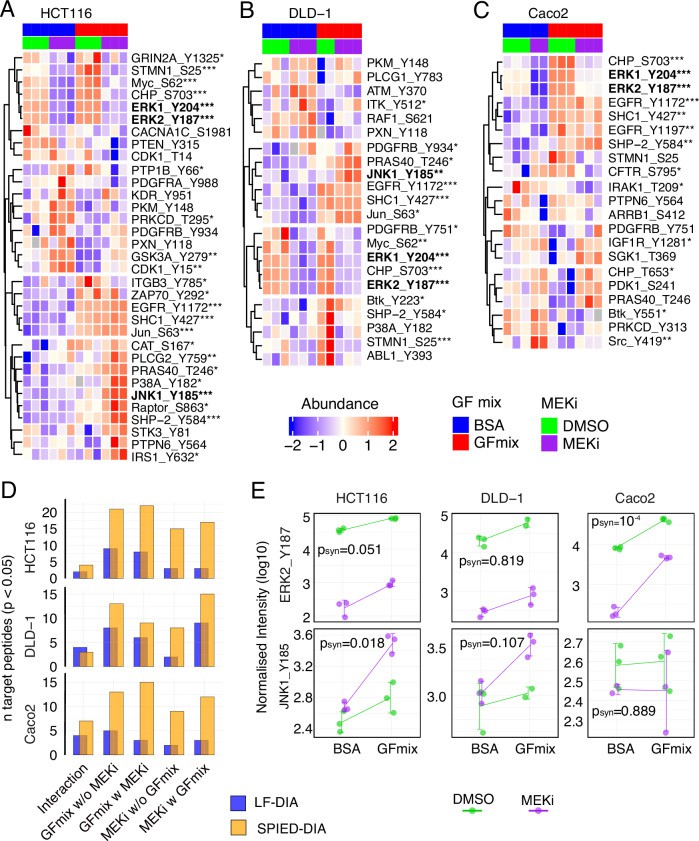 SPIED-DIA analysis of targeted regulated phosphorylation sites (van Bentum M et al/. 2025)
SPIED-DIA analysis of targeted regulated phosphorylation sites (van Bentum M et al/. 2025)
Case 2: SERCA2 Regulatory Mechanism Analysis
Methodological Framework
- Phosphosite Identification
- LC-MS/MS analysis revealed GSK3β-dependent phosphorylation at SERCA2-S663
- Generated phospho-specific anti-S663 antibody validated through λ phosphatase sensitivity testing
- Functional Validation Model
- Engineered S663A (phospho-ablative) and S663E (phosphomimicry) mutants via CRISPR-Cas9
- Delivered mutants to cardiomyocytes using AAV9 vectors (1×10¹¹ PFU dose)
- In vitro kinase assays confirmed GSK3β-mediated direct phosphorylation (2.8-fold Vmax increase)
Experimental Findings
- Pathological Regulation Mechanism
- Ischemia-reperfusion injury triggers GSK3β activation, elevating SERCA2-S663 phosphorylation 3-fold
- Phosphorylation inhibits SERCA2 activity (40% reduced Ca²⁺ uptake) and induces cytoplasmic Ca²⁺ overload
- Therapeutic Interventions
- S663A mutant demonstrated:
- 58% reduction in myocardial infarction area (p<0.01)
- Improved mitochondrial function (35% decreased ROS generation)
- Clinical correlation: Cardiac tissue S663 phosphorylation inversely associates with ejection fraction in heart failure (r=-0.72)
Technical Innovations
- Live-cell Ca²⁺ imaging (Fluo-4 AM) showed S663A mutant accelerates sarcoplasmic reticulum Ca²⁺ recovery (28% reduced τ)
- GSK3β inhibitors targeting S663 phosphorylation (e.g., TDZD-8) mitigate ischemia-reperfusion injury (Gonnot F et al., 2023)
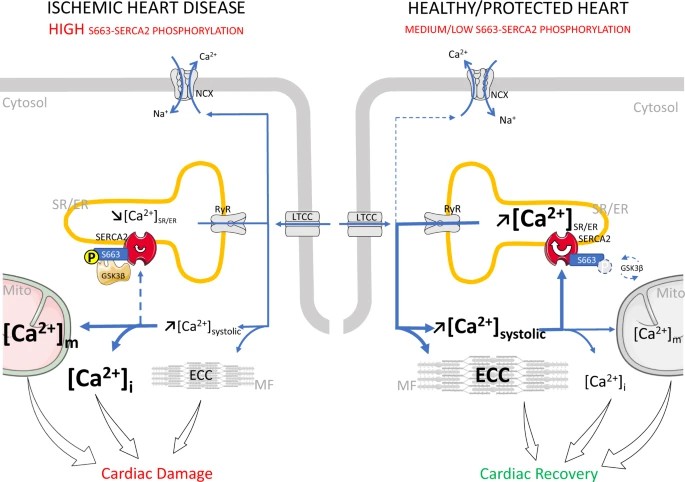 Mechanistic role of SERCA2 phosphorylation at S663 in ischemic and healthy/protected hearts (Gonnot F et al., 2023)
Mechanistic role of SERCA2 phosphorylation at S663 in ischemic and healthy/protected hearts (Gonnot F et al., 2023)
Case 3: Regulatory Mechanism of PPARγ Phosphorylation and Development of Novel Ligand UHC1
Core Discoveries
- PPARγ Phosphorylation Regulation
- CDK5 mediates site-specific phosphorylation at PPARγ-Ser273, exacerbating inflammatory responses and insulin resistance
- Phosphorylation enhances PPARγ's interaction with proinflammatory transcription factors (e.g., NF-κB)
- Novel Ligand Development
- Structural Optimization: Modified SR1664 derivative UHC1 exhibits improved solubility (ΔLogP=-1.2) and binding affinity (KD=38 nM vs. SR1664 120 nM)
- Pharmacological Characteristics:
- Maintains non-agonist property (lacks classical PPARγ transactivation)
- Selectively inhibits CDK5-dependent Ser273 phosphorylation (85% inhibition, IC50=0.8 μM)
Experimental Validation
- Biophysical Characterization
- TR-FRET analysis confirmed competitive binding to PPARγ-LBD (Ki=42 nM)
- SPR demonstrated extended target engagement (t1/2=18 min)
- Functional Efficacy
- In vitro kinase assays: UHC1 blocks CDK5/p35-mediated phosphorylation (3-fold Vmax reduction)
- In vivo diabetic models:
- Significant hypoglycemia (32% fasting glucose reduction)
- Potent anti-inflammatory activity (65% TNF-α decrease) without weight gain
Translational Value
- Circumvents adverse effects of conventional TZDs (e.g., edema)
- Completed preclinical safety assessment (NOAEL=100 mg/kg)
Conclusion: UHC1 represents a novel phosphorylation-targeting therapeutic strategy for diabetes management (Choi SS et al., 2014).
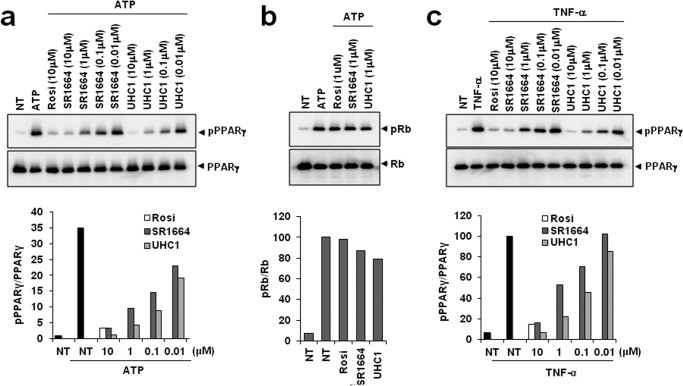 Inhibition of CDK5-mediated PPARγ phosphorylation by UHC1 (Choi SS et al., 2014)
Inhibition of CDK5-mediated PPARγ phosphorylation by UHC1 (Choi SS et al., 2014)
To learn more about phosphoproteomics, please refer to "Phosphoproteomics in Cancer Research: A Data-Driven Approach".
References
- Agrotis A, Lamoliatte F, Williams TD, Black A, Horberry R, Rousseau A. "Multiple phosphorylation of the Cdc48/p97 cofactor protein Shp1/p47 occurs upon cell stress in budding yeast." Life Sci Alliance. 2023 Jan 24;6(4):e202201642. doi: 10.26508/lsa.202201642
- Han T, Wang Y, Cheng M, Hu Q, Wan X, Huang M, Liu Y, Xun W, Xu J, Wang L, Luo R, Yuan Y, Wang K, Wang J. "Phosphorylated SHMT2 Regulates Oncogenesis Through m6A Modification in Lung Adenocarcinoma." Adv Sci (Weinh). 2024 May;11(18):e2307834. doi: 10.1002/advs.202307834
- Yasukawa M, Ando Y, Yamashita T, Matsuda Y, Shoji S, Morioka MS, Kawaji H, Shiozawa K, Machitani M, Abe T, Yamada S, Kaneko MK, Kato Y, Furuta Y, Kondo T, Shirouzu M, Hayashizaki Y, Kaneko S, Masutomi K. "CDK1 dependent phosphorylation of hTERT contributes to cancer progression." Nat Commun. 2020 Mar 25;11(1):1557. doi: 10.1038/s41467-020-15289-7
- Chen Y, Dufour CR, Han L, Li T, Xia H, Giguère V. "Hierarchical Phosphorylation of HOXB13 by mTOR Dictates Its Activity and Oncogenic Function in Prostate Cancer." Mol Cancer Res. 2023 Oct 2;21(10):1050-1063. doi: 10.1158/1541-7786.MCR-23-0086
- van Bentum M, Klinger B, Sieber A, Naghiloo S, Zauber H, Lehmann N, Haji M, Niquet S, Mertins P, Blüthgen N, Selbach M. "Spike-in enhanced phosphoproteomics uncovers synergistic signaling responses to MEK inhibition in colon cancer cells." Nat Commun. 2025 May 27;16(1):4884. doi: 10.1038/s41467-025-59404-y
- Gonnot F, Boulogne L, Brun C, Dia M, Gouriou Y, Bidaux G, Chouabe C, Crola Da Silva C, Ducreux S, Pillot B, Kaczmarczyk A, Leon C, Chanon S, Perret C, Sciandra F, Dargar T, Gache V, Farhat F, Sebbag L, Bochaton T, Thibault H, Ovize M, Paillard M, Gomez L. "SERCA2 phosphorylation at serine 663 is a key regulator of Ca2+ homeostasis in heart diseases." Nat Commun. 2023 Jun 8;14(1):3346. doi: 10.1038/s41467-023-39027-x
- Choi SS, Kim ES, Koh M, Lee SJ, Lim D, Yang YR, Jang HJ, Seo KA, Min SH, Lee IH, Park SB, Suh PG, Choi JH. "A novel non-agonist peroxisome proliferator-activated receptor γ (PPARγ) ligand UHC1 blocks PPARγ phosphorylation by cyclin-dependent kinase 5 (CDK5) and improves insulin sensitivity." J Biol Chem. 2014 Sep 19;289(38):26618-26629. doi: 10.1074/jbc.M114.566794














