Phosphoproteomics constitutes a specialized proteomic discipline focused on comprehensive characterization of protein phosphorylation events. Utilizing mass spectrometry (MS), this technique enables both qualitative identification and quantitative measurement of phosphopeptides. Recent MS innovations, particularly Parallel Accumulation-Serial Fragmentation coupled with Trapped Ion Mobility Spectrometry (TIMS), have empowered deep phosphoproteome characterization at high coverage levels. Such methodological advancements significantly enhance acquisition velocity and analytical precision, permitting robust phosphosite mapping within complex tumor matrices.
Cancer phosphoproteomics employs a data-centric paradigm that comprehensively investigates tumorigenesis, metastatic progression, and therapy resistance mechanisms. This approach integrates three critical components: high-fidelity phosphosite mapping, multi-omics data synthesis, and artificial intelligence-driven modeling. Its fundamental objective transforms extensive phosphorylation datasets into clinically actionable biological intelligence for therapeutic decision support.
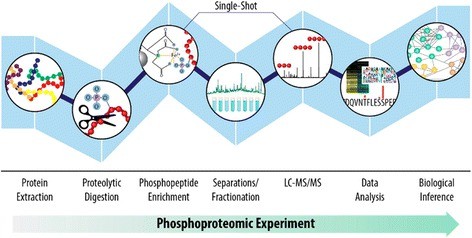 Typical phosphoproteomics workflow (Gruber W et al., 2017)
Typical phosphoproteomics workflow (Gruber W et al., 2017)
Strategic value of cancer phosphorylation network
Phosphorylation networks hold strategic importance in oncology, functioning as central signaling hubs that coordinate >80% of malignancy-associated pathways—including RTK, MAPK, and PI3K-AKT cascades. These networks represent fundamental drivers of tumor heterogeneity, with phosphorylation dysregulation frequently preceding genomic mutations chronologically. Therapeutically, they constitute prime targets evidenced by kinase inhibitors comprising 45% of FDA-approved cancer drugs (2023 data). Notably, specific phosphosites serve as clinical biomarkers for therapeutic resistance, exemplified by EGFR T790M phosphorylation.
- Key technical challenges in cancer phosphoproteomics include:
- Dynamic range compression: Kinase signaling represents<0.1% of total cellular protein (phosphotyrosine comprises merely 0.05% of phosphomodifications)
- Sample limitations: Needle biopsies (<1mg) require amol-level detection (e.g., timsTOF SCP: 5-20 amol)
- Localization ambiguity: Multiply phosphorylated peptides exhibit variable identification rates (Orbitrap EThcD >85% vs. conventional CID 50-65%)
- These constraints are addressed through an integrated workflow combining:
- Targeted phosphoenrichment
- Intelligent acquisition algorithms
- Cloud-based computational analytics
To learn more about the mechanism and function of phosphorylation, please refer to "Understanding Protein Phosphorylation: Mechanisms and Biological Roles".
Experimental Design Optimization
1. Sample Pretreatment
- Enrichment Strategy Validation
- Tissue biopsies (>10 μg): TiO₂/MOAC achieves >85% phosphopeptide recovery
- Limited samples (<1 μg): Anti-pY pre-enrichment yields 50-fold detection gain
- Liquid biopsy CTC exosomes: pS1265 detection limit = 1 pg/mL via phosphoprotein-capture chip
2. Mass Spectrometry Acquisition Parameters
Sample Size Thresholds
- ≥100 cells:
- Mode: Data Independent Acquisition (DIA)
- Platforms: SWATH® (TripleTOF) or ion mobility–enabled DIA acquisition mode (timsTOF)
- Window optimization: Dynamic m/z partitioning (400-900 Da)
- Benefit: >30% increased phosphopeptide coverage
- ≤100 cells:
- Mode: Data Dependent Acquisition (DDA)
- Fragmentation: Primary EThcD (electron-transfer/high-energy collision dissociation)
- Neutral loss triggering: Monitors -79.97/-97.97 Da events
- Auto-switch: Activates EThcD upon neutral loss detection
- Default: High-energy collision dissociation (HCD) when untriggered
Dynamic Window Configuration
- Isolation window: 8-25 Da (precursor density-dependent)
- Non-overlapping design: Asymmetric windows (e.g., 400-425, 424-450)
- Efficiency: 40% increase in phosphopeptide IDs for complex specimens
Sample-Type Specific Parameters
- Tissue sections (DIA): 40-60 windows
- Single-cell/trace samples: DDA top 20 MS/MS
- Exosomal phosphoproteins: Hybrid DDA-DIA quantitation
3. Data Analysis Framework
Phosphorylation Signal Processing
- Preprocessing
- Raw data: MaxQuant/Proteome Discoverer
- Site localization: PTMProphet (FDR<1%)
- Functional Annotation
- Kinase networks: PhosphoSitePlus® with substrate prediction
- Pathway mapping: Reactome phospho-specific databases
- Clinical Correlation
- Survival: Cox regression models correlate site-specific phosphorylation with OS/PFS
- Therapy response: Random forest predictor (AUC >0.85)
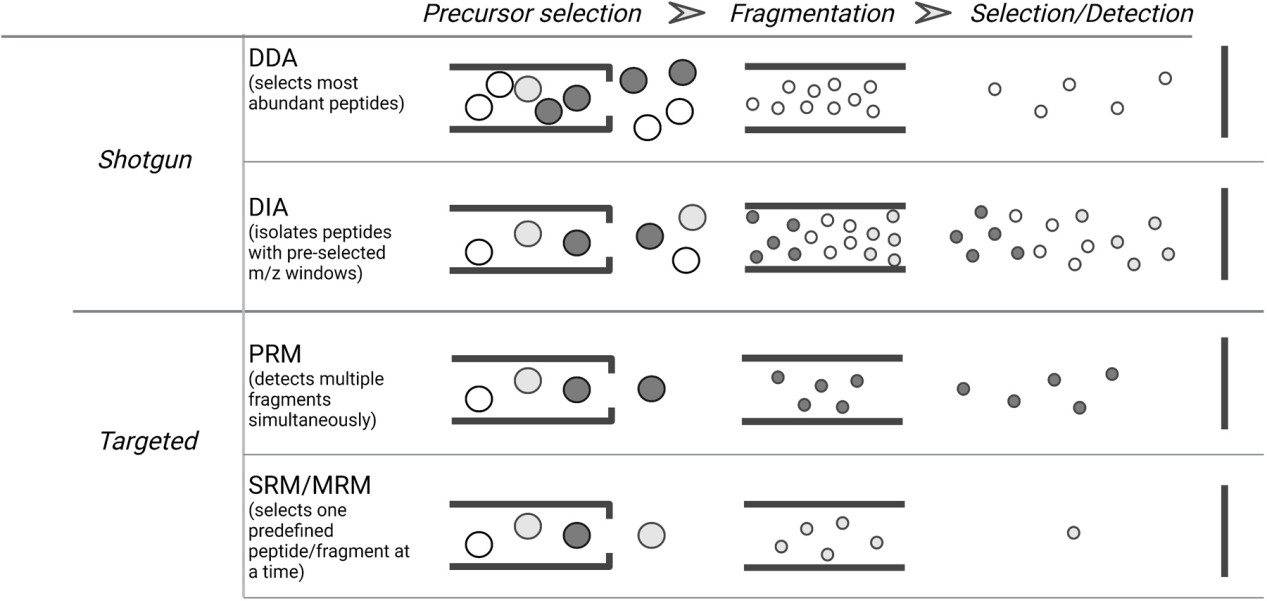 Types of MS data acquisition modes in bottom-up proteomics (Gerritsen JS et al., 2021)
Types of MS data acquisition modes in bottom-up proteomics (Gerritsen JS et al., 2021)
For more detailed workflow, please refer to "Phosphoproteomics Workflow Explained: From Sample to Data".
Data-Centric Research Architecture
1. Innovative Sampling Approaches
Data-optimized specimen processing
- Circulating tumor cell (CTC) metastasis evolution: Real-time monitoring via timsTOF SCP + microfluidic enrichment
- Non-invasive liquid biopsies: High-depth exosomal phosphoproteomics using Orbitrap DIA
- Spatial phosphoproteomics: Tumor microenvironment interactions mapped at 10μm resolution via imaging mass spectrometry
2. Advanced Detection Technologies
Comprehensive profiling systems
- Deep coverage:
- Orbitrap Eclipse: >240,000 phosphosites/sample (>120min LC gradient)
- 4D-DIA (timsTOF): Ion mobility (CCS values) enhances localization precision
- Dynamic monitoring:
- ¹⁵N metabolic labeling + TMT multiplexing: Drug resistance trajectory tracking
3. AI-Driven Analytical Engine
Multimodal Network Modeling
- Kinase activity: NetPhorest phosphosite interrogation
- Multi-omics integration: DeepKinase fusion of phospho/mutation/transcriptome data
- Key target identification: HITS algorithm prioritizes core drivers
Core Computational Modules
- Functional mapping: Kinase-substrate networks (PhosphoNET/Kinase Phos 2.0)
- Resistance prediction: ResNet-Cancer graph neural networks
- Biomarker discovery: XGBoost-SHAP feature interpretation for therapy response
4. Clinical Validation Workflow
Predictive model → PDX efficacy assessment → Patient-derived organoid testing → Clinical trial cohort stratification
Key Technological Innovations
| Domain | Advancement | Performance |
|---|---|---|
| CTC Analysis | Microfluidic chip enables single-CTC isolation | 300+ phosphosites/cell (timsTOF SCP) |
| 4D-DIA Advantage | Adds ion mobility dimension → 5× resolution gain vs traditional 3D-DIA (m/z, RT, intensity) | |
| Resistance Tracking | ¹⁵N-labeled cells + TMT-mixed resistant/sensitive specimens → phosphodynamic quantification | Detects<5% phosphorylation variation in resistant subpopulations |
| ResNet-Cancer Model | Input: Phospho-network + drug features → Output: Resistance probability score (AUC=0.92) |
Framework Implementation Benefits
- Phosphorylation-driven event mapping: 2-4 weeks
- AI-prioritized target list (TOP10): 1 week
- Organoid therapy response prediction: >85% accuracy (3-week verification)
Select Service
Learn more
Breakthrough Applications in Cancer Phosphoproteomics
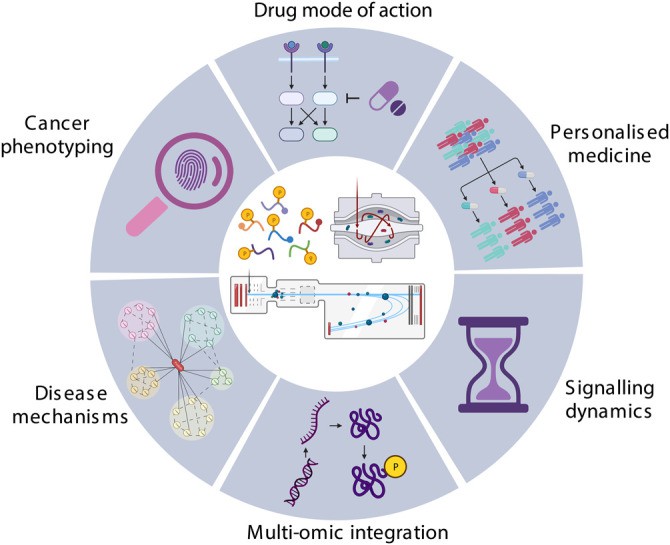 Application of phosphoproteomics in cancer research (Higgins L et al., 2023)
Application of phosphoproteomics in cancer research (Higgins L et al., 2023)
1. Molecular Pathogenesis Insights
Phosphoproteomic profiling uncovers signaling dysregulation in malignancies, particularly imbalances in cell cycle control, apoptosis evasion, proliferative signaling, and migratory pathways. e.g., Phosphoproteomic quantification in ovarian cancer reveals L1CAM as a driver of cancer stemness and radioresistance. This occurs through orchestrated phosphorylation of critical effectors, including DNA repair machinery and mTORC1 signaling components. Functional validation confirms L1CAM ablation: Suppresses anchorage-independent growth and clonogenic capacity; Diminishes in vivo tumorigenicity. These results establish L1CAM-directed phosphoreprogramming as the central mechanism sustaining malignant progression and therapy resistance (Todorov TZ et al., 2025).
2. Therapeutic Resistance Mechanisms
Comparative phosphodynamic analysis pre- versus post-treatment identifies resistance-associated phosphosignatures, elucidating escape mechanisms and informing novel therapeutic target discovery. e.g., Quantitative phosphoproteomics reveals core mechanisms underlying acquired resistance to tyrosine kinase inhibitors: Resistance-Specific Signatures; Pazopanib resistance: Hyperphosphorylation of cytoskeletal regulatory pathways; Dasatinib resistance: Elevated phospho-activation of insulin/IGF-1R signaling. Phosphoproteomic Remodeling Scope: Drug exposure altered only 6% (pazopanib) and 9.7% (dasatinib) of phosphosites, indicating targeted signaling rewiring rather than global dysregulation. Therapeutic Intervention: HSP90 inhibitor NVP-AUY-922 overcomes resistance by: Blocking HSP90 chaperone function; Disrupting resistance-associated phosphonetworks (Vyse S et al., 2018).
3. Metastatic Regulation
This approach delineates key phosphorylation mediators of metastasis by contrasting phosphoprofiles between primary and disseminated tumor cells, revealing invasion-migration biomarkers. e.g., Phosphoproteomic quantification reveals that biliary tract cancer cells treated with LAT1 inhibitor JPH203 undergo rapid depletion of essential amino acids. This triggers phosphosignaling reprogramming characterized by: Downregulation of cell cycle regulators; Reduced phosphorylation of RNA splicing machinery. Crucially, JPH203 impairs CK2 substrate recognition by disrupting kinase-regulatory protein interactions (CK2–NOLC1), rather than direct enzymatic inhibition. This allosteric mechanism inactivates CK2-mediated oncogenic signaling. Therapeutic Synergy: Combined JPH203 and CK2 inhibition synergistically suppresses biliary cancer proliferation and migration, establishing molecular rationale for targeting the LAT1–CK2 axis (Okanishi H et al., 2022).
4. Tumor Microenvironment Dynamics
Mapping phosphorylation events within tumor-stroma interactions exposes immune evasion tactics and microenvironmental remodeling, advancing immunotherapy and stroma-targeted strategies. e.g., Chemical proteomics and phosphoproteomics were combined to delineate how multi-kinase inhibitors concurrently target malignant cells and microenvironment in lung cancer. Key findings include:Global Target Landscape, Identification of >100 kinase targets and critical complexes (e.g., AMPK/TBK1, ILK); Microenvironment-Specific Targets, Discovery of tumor stroma-selective kinase interactions; Transcompartmental Pathway Inhibition, Concurrent suppression of MAPK, integrin, and immune signaling across cellular compartments. This panoramic profiling of multi-target drug action in dual tumor microdomains informs rational combination therapy design (Gridling M et al., 2014).
5. Clinical Prognostication
Phosphomarker dynamics correlate with patient survival outcomes, recurrence likelihood, and metastatic potential, enabling treatment response prediction and precision therapeutic stratification. e.g., Integrated proteomic and phosphoproteomic profiling identifies RAI14 as an APC mutation-specific prognostic biomarker in colorectal cancer. Elevated RAI14 expression correlates with diminished chemotherapy efficacy and poorer survival outcomes. Mechanistically, RAI14 drives tumor invasion by reconfiguring adhesion-associated phosphonetworks to promote epithelial-mesenchymal transition (EMT) and stabilize F-actin. This discovery establishes RAI14 as both an early-warning indicator and novel therapeutic target for APC-mutant CRC patients (Zhang R et al., 2023).
6. Precision Oncology Framework
Cancer phosphoproteomics facilitates personalized intervention through differential phosphorylation mapping to pinpoint driver events. Concurrently, it accelerates targeted drug development against pathological phosphoproteins. e.g., Addressing the need for personalized therapy in metastatic castration-resistant prostate cancer (mCRPC), targeted phosphoproteomics integrated with mass spectrometry offers a solution to current clinical detection constraints. These limitations include genomic analysis blind spots and the restricted throughput inherent in antibody-based techniques. This approach enables the high-efficiency identification of thousands of phosphopeptide biomarkers. Technical Advantages: Compared to conventional methodologies, targeted mass spectrometry delivers significantly enhanced reproducibility, superior sensitivity, and faster processing times, while also lowering costs associated with clinical implementation. Application Scenarios: The technique is applicable to prostate cancer cell lines and clinical tissue samples, driving target discovery, mechanistic drug resistance analysis, and precise patient stratification. Clinical Value: It provides a novel approach for developing individualized treatment strategies, ultimately aiming to extend survival and improve quality of life for mCRPC patients (Ramroop JR et al., 2018).
Strategic Technical Development Focus
1. Single-Cell Phosphoproteomics
- Challenge: Phosphopeptide detection rates<0.1% at single-cell level
- Solution: Implementation of Astral analyzers (0.01 amol sensitivity threshold)
2. Post-Translational Modification Crosstalk
- Methodology: Phospho-ID platform enabling phosphorylation-ubiquitination interplay capture
3. In Vivo Dynamics Monitoring
- Innovation: Emerging nanoprobe PET-CT platforms with ⁶⁸Ga-labeled phosphopeptide tracers
Data Resource Platform
| Database | Core Value | Link |
|---|---|---|
| CPTAC-Cancer | >10,000 cancer phosphoproteome cases | cptac-data.org |
| PhosphoSitePlus Cancer | Curated oncogenic phosphorylation sites | phosphosite.org |
| DeepPhos-Cancer | AI-predicted anti-cancer targets | deepkinase.org |
Cancer Phosphoproteomics: Transition to High-Dimensional Data-Driven Clinical Integration
This field has overcome critical technical barriers and now operates within a high-dimensional data-driven paradigm, with transformative clinical applications:
- Diagnostic Transformation: Spatial phosphoproteomic profiling is redefining pathological classification standards
- Therapeutic Guidance: Phosphorylation network mapping directs precision combination therapies
- Monitoring Advancements: Exosomal phosphofingerprint analysis enables non-invasive early detection
Recent breakthroughs in Astral mass spectrometry (2024) and single-cell algorithms are accelerating clinical adoption, positioning phosphoproteomics to fundamentally reshape oncology practice guidelines.
References
- Gruber W, Scheidt T, Aberger F, Huber CG. "Understanding cell signaling in cancer stem cells for targeted therapy - can phosphoproteomics help to reveal the secrets?" Cell Commun Signal. 2017 Mar 29;15(1):12. doi: 10.1186/s12964-017-0166-1
- Gerritsen JS, White FM. "Phosphoproteomics: a valuable tool for uncovering molecular signaling in cancer cells." Expert Rev Proteomics. 2021 Aug;18(8):661-674. doi: 10.1080/14789450.2021.1976152
- Higgins L, Gerdes H, Cutillas PR. "Principles of phosphoproteomics and applications in cancer research." Biochem J. 2023 Mar 31;480(6):403-420. doi: 10.1042/BCJ20220220
- Todorov TZ, Coelho R, Jacob F, Heinzelmann-Schwarz V, Schibli R, Béhé M, Grünberg J, Grzmil M. "Phosphoproteomics Reveals L1CAM-Associated Signaling Networks in High-Grade Serous Ovarian Carcinoma: Implications for Radioresistance and Tumorigenesis." Int J Mol Sci. 2025 May 10;26(10):4585. doi: 10.3390/ijms26104585
- Okanishi H, Ohgaki R, Xu M, Endou H, Kanai Y. "Phosphoproteomics revealed cellular signals immediately responding to disruption of cancer amino acid homeostasis induced by inhibition of L-type amino acid transporter 1." Cancer Metab. 2022 Nov 10;10(1):18. doi: 10.1186/s40170-022-00295-8
- Vyse S, McCarthy F, Broncel M, Paul A, Wong JP, Bhamra A, Huang PH. "Quantitative phosphoproteomic analysis of acquired cancer drug resistance to pazopanib and dasatinib." J Proteomics. 2018 Jan 6;170:130-140. doi: 10.1016/j.jprot.2017.08.015
- Gridling M, Ficarro SB, Breitwieser FP, Song L, Parapatics K, Colinge J, Haura EB, Marto JA, Superti-Furga G, Bennett KL, Rix U. "Identification of kinase inhibitor targets in the lung cancer microenvironment by chemical and phosphoproteomics." Mol Cancer Ther. 2014 Nov;13(11):2751-62. doi: 10.1158/1535-7163.MCT-14-0152
- Zhang R, Hu M, Chen HN, Wang X, Xia Z, Liu Y, Wang R, Xia X, Shu Y, Du D, Meng W, Qi S, Li Y, Xu H, Zhou ZG, Dai L. "Phenotypic Heterogeneity Analysis of APC-Mutant Colon Cancer by Proteomics and Phosphoproteomics Identifies RAI14 as a Key Prognostic Determinant in East Asians and Westerners." Mol Cell Proteomics. 2023 May;22(5):100532. doi: 10.1016/j.mcpro.2023.100532
- Ramroop JR, Stein MN, Drake JM. "Impact of Phosphoproteomics in the Era of Precision Medicine for Prostate Cancer." Front Oncol. 2018 Feb 16;8:28. doi: 10.3389/fonc.2018.00028











