Phosphoproteomics research plays a vital role in elucidating cellular signaling pathways and disease pathogenesis, where data acquisition methodologies critically determine experimental success. Currently, the two dominant approaches—Data-Dependent Acquisition (DDA) and Data-Independent Acquisition (DIA)—each offer distinct advantages. This study targets professional target customers, providing a detailed examination of DIA and DDA technical distinctions and practical performance in phosphoproteomic applications. Our comparative analysis empowers informed selection of optimal analytical strategies.
1. Foundational Principles and Workflows
DDA Methodology
- Acquisition Mechanism: Dynamically selects precursor ions based on signal intensity. Following full MS1 scans, the most abundant ions (e.g., top 20) undergo fragmentation (MS2), with iterative cycling between MS1 and MS2 scans.
- Phosphoproteome Enhancement: Implements HCD-triggered acquisition to boost phosphopeptide sensitivity through preferential fragmentation of phosphorylation-indicative precursors. Complementary "Multi-notch" strategies concurrently fragment multiple precursors to expand coverage.
- Standardized Workflow:
- Sample preparation (protein extraction/enzymatic digestion)
- LC-MS/MS with DDA acquisition
- Database searching via MaxQuant/Proteome Discoverer
- Phosphosite localization/quantification requiring comprehensive fragment ion coverage
- Advantages: Mature automated processing ideal for phosphoproteomic characterization.
DIA Methodology
- Acquisition Mechanism: Partitions m/z ranges into fixed isolation windows (typically 24-32), synchronously fragmenting all precursors within each window without real-time selection. This non-targeted approach enables large-scale complex sample quantification.
- Phosphoproteome Specificity: Relies on high-resolution fragment ion analysis coupled with phospho-specific spectral libraries, utilizing reference fragmentation patterns to extract quantitative ion pairs from multiplexed spectra.
- Integrated Workflow:
- Equivalent sample preparation to DDA
- Construction of high-quality spectral libraries (DDA-generated or hybrid)
- LC-MS/MS with DIA acquisition
- Library-dependent deconvolution using Spectronaut/DIA-NN/Skyline (or library-free approaches)
- Quantification via spectral library matching for precise phosphosite assignment
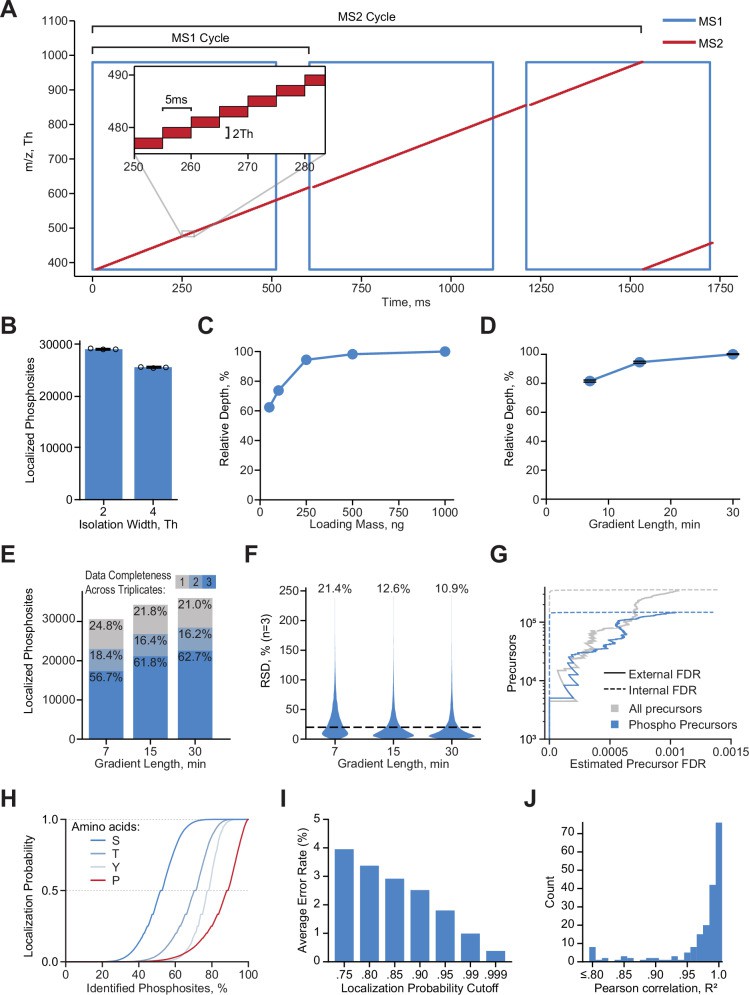 A Illustration of DIA acquisition scheme (Lancaster NM et al., 2024)
A Illustration of DIA acquisition scheme (Lancaster NM et al., 2024)
2. Core Performance Dimensions Comparison
DDA
Coverage Limitations
- Ion Selection Bias: Prioritizes high-abundance precursors for fragmentation, creating significant ion suppression effects. This abundance-dependent selection disadvantages low-abundance phosphopeptides, particularly in complex samples (e.g., whole-cell lysates).
- Identification Depth: Typically detects 2,000-4,000 phosphosites per run, suitable for small-scale studies but exhibits instability in complex matrices.
- Technical Reproducibility: Subject to substantial batch-to-batch variability due to stochastic precursor selection. Inconsistent fragmentation across runs reduces analytical reliability.
Quantitative Performance
- Methodology: Relies on MS1 precursor intensity, constrained by selective ion sampling and fragment ion abundance.
- Data Completeness: Frequent missing values (>20% CV common), especially for low-abundance phosphosites, compromising statistical power.
- Application Constraints: Limited suitability for clinical/large-cohort studies due to reproducibility challenges and data dropouts.
DIA
Coverage Advantages
- Unbiased Acquisition: Synchronously fragments all precursors within fixed m/z windows (typically 24-32), eliminating ion suppression artifacts.
- Enhanced Detection: Improves phosphopeptide identification by 30-100% versus DDA, routinely detecting 8,000-12,000 high-confidence phosphosites per run.
- Run-to-Run Consistency: Near-complete data collection ensures high technical reproducibility (CV <15-20%), ideal for longitudinal clinical studies.
Quantitative Precision
- Comprehensive Quantification: Minimal missing values through signal extraction from all acquired spectra, preserving data integrity.
- Alignment Algorithms: Tools like DIA-NN correct retention time drift, enhancing quantification accuracy.
- Cohort Analysis Strength: Superior suitability for large-scale biomarker discovery and drug response studies requiring high quantitative consistency.
Novel Phosphosite Discovery Capabilities
DDA Methodology
- Advantages:
- Excels in identifying uncharacterized phosphorylation events through high-quality MS/MS spectra
- Enables direct database searching for site localization via diagnostic fragment ions (e.g., phosphotyrosine immonium ions) and neutral loss patterns
- Provides definitive evidence for rare modifications without prior spectral references
- Limitations:
- Low-abundance phosphopeptides often evade detection due to dynamic exclusion
- Requires manual spectrum validation, increasing analytical burden
DIA Methodology
- Advantages:
- Delivers comprehensive coverage of pre-characterized phosphosites in large cohorts
- Maintains consistent detection across biological replicates
- Limitations:
- Restricted to modifications represented in spectral libraries
- Emerging library-free approaches (e.g., DIA-NN) show promise but require validation
- Precise site assignment remains dependent on reference fragmentation patterns
Analytical Confidence & Data Quality
DDA Performance
- Spectrum Quality: Unambiguous precursor-fragment relationships facilitate visual verification
- Downstream Strengths: Simplified site localization supports targeted investigations
- Data Gaps: Inherent selection bias creates coverage limitations
DIA Performance
- Spectral Complexity: Multiplexed fragmentation demands advanced deconvolution algorithms
- Downstream Requirements: Dependent on computational tools (DIA-NN, Skyline) with machine learning capabilities
- Compensating Advantage: Near-complete data acquisition enhances quantitative rigor
Instrumentation & Economic Considerations
DDA Implementation
- Hardware Flexibility: Compatible with mid-tier platforms (Orbitrap Exploris™, Q-TOF)
- Cost Analysis: Lower capital investment offset by need for technical replicates
DIA Implementation
- System Requirements: Requires high-resolution instruments (Q-Exactive™ HF-X, timsTOF® SCP)
- Economic Efficiency: Higher upfront costs balanced by reduced replicate needs in large studies
Operational Throughput
DDA Workflows
- Throughput Constraints: Repeated runs needed to compensate for stochastic sampling
- Optimal Use Cases: Hypothesis-driven studies targeting specific phosphosites
DIA Workflows
- Acquisition Efficiency: Single-injection comprehensive data capture
- Scalability: Superior for longitudinal clinical studies requiring batch consistency
3. Key Pain Points and Solutions
| Pain Points | DDA Strategy | DIA Strategy | CRO/Lab Implementation Recommendations |
|---|---|---|---|
| Coverage vs. Reproducibility Trade-off | Sacrifice quantitative reproducibility for depth (e.g., reduce Top N) | Simultaneously achieve high reproducibility and depth | Prioritize DIA: For cohorts ≥10 samples, DIA outperforms in coverage/reproducibility, reducing downstream costs. |
| Low-Abundance PTM Detection | Boost sensitivity via high loading, pre-fractionation, chemical enrichment | Non-selective acquisition enhances low-abundance signal capture | Combine DDA + DIA: Use DDA for deep spectral library building; deploy DIA for cohort quantification. Alternatively, leverage DIA with advanced denoising algorithms. |
| Confident Phosphosite Localization | Employ ETD/ECD fragmentation; manual MS/MS validation | Require high-quality spectral libraries + strict filtering (e.g., DIA-NN PEP <0.01) | Mandatory verification: Critical sites need orthogonal validation (e.g., PRM, site-directed mutagenesis) regardless of method. |
| Lack of Phospho-Specific Libraries | / | Pre-run DDA for custom libraries; public databases (e.g., PhosphoSitePlus); hybrid libraries | Allocate budget for library construction: Dedicated phospho-specific libraries are essential for large-scale DIA projects. |
| High DIA Data Complexity | / | Dependency on specialized tools (Spectronaut, DIA-NN) + bioinformatics support | Standardize workflows: Train staff on mainstream software; establish optimized pipelines. Allocate high-performance computing resources for large projects. |
4. Strategic Selection: Optimizing Methodologies for Research Objectives
DIA Implementation Framework
- Optimal Use Cases:
- Large Clinical Cohorts: Ideal for phosphoproteomic analyses involving >10 samples, particularly in therapeutic monitoring and biomarker verification requiring high quantitative precision
- Complex Matrices: Superior performance in tissue/plasma samples where comprehensive coverage is essential
- Longitudinal Studies: Maintains temporal data integrity for multi-timepoint comparisons
- Key Advantages:
- Unparalleled quantitative reproducibility in large-scale analyses
- Enhanced detection of low-abundance phosphosites
- Consistent performance across extended study timelines
- Implementation Requirements:
- Established phospho-specific spectral libraries
- High-performance instrumentation (e.g., Q-Exactive HF-X)
- Specialized bioinformatics capabilities for DIA data processing
DDA Implementation Framework
- Optimal Use Cases:
- Exploratory Investigations: Small-scale studies (<10 samples) targeting novel phosphosite discovery
- Resource-Constrained Settings: Viable alternative when DIA infrastructure is unavailable
- Validation-Centric Projects: Where manual spectrum verification is prioritized
- Methodological Strengths:
- Direct identification of uncharacterized phosphorylation events
- Operational readiness with established analytical workflows
- Inherent Constraints:
- Limited quantitative precision in cohort studies
- Reduced sensitivity for low-abundance modifications
Integrated DDA-DIA Approach
- Strategic Applications:
- Translational Research: Combines initial discovery with large-scale validation
- Resource-Efficient Studies: Maximizes output from limited sample availability
- Implementation Protocol:
- Library Generation: Conduct deep-coverage DDA runs to build spectral references
- Cohort Quantification: Perform DIA acquisition across all samples
- Biological Interpretation: Conduct network/pathway analyses using DIA-derived quantitative data
- Operational Benefits:
- Balanced discovery-validation capabilities
- Adaptability to complex biological questions (e.g., drug mechanism studies)
Comparative Summary
| Technology | Best Suited Scenarios | Scenarios to Avoid |
|---|---|---|
| DDA | 1. Discovery of novel phosphorylation sites 2. Mechanism exploration with low sample size 3. Low-abundance modification validation (after fractionation) | Large cohort quantitative studies, Longitudinal analysis of clinical samples |
| DIA | 1. Quantitative studies for cohorts of 50+ samples 2. Low-input samples (e.g., biopsy specimens) 3. Dynamic tracking of phosphorylation signaling pathways | Projects without reference spectral libraries and no budget for library generation |
| Hybrid (DDA/DIA) | 1. Deep coverage + quantification with limited samples 2. CRO platform services (one library for multiple uses) | Simple low-throughput needs (introduces unnecessary complexity) |
Select Service
Learn more
5. Classic Case
DDA Implementation Case Study
Case 1
Research Objective: Develop pHASED phosphoproteomics to elucidate AML therapeutic resistance mechanisms
Technical Platform: FAIMS-Orbitrap Exploris 480 DDA workflow
Experimental Design
- Cell Models:
- Isogenic FDC-P1 AML lines: FLT3-ITD, FLT3-ITD/D835V, FLT3-ITD/D835Y
- Culture: Factor-depleted DMEM (10% FBS), mycoplasma-free
- Phosphoproteomic Workflow:
- Lysis: Alkaline disruption (0.1M Na₂CO₃, pH 11.3) + ultrasonication
- Digestion: Sequential LysC/trypsin cleavage
- Enrichment: TiO₂ phosphopeptide capture
- LC-MS/MS:
- Chromatography: 75-min ACN gradient (nanoLC-C18)
- FAIMS: Multi-CV optimization (-70, -60, -50, -40 V)
- MS: Orbitrap Exploris 480; Res: MS1 60k, MS2 15k
- Data Processing:
- Search: Proteome Discoverer 2.4 (UniProt mouse + FLT3 mutants; FDR<1%)
- Quantification: Heavy-labeled standard normalization → log₂ differential phosphorylation
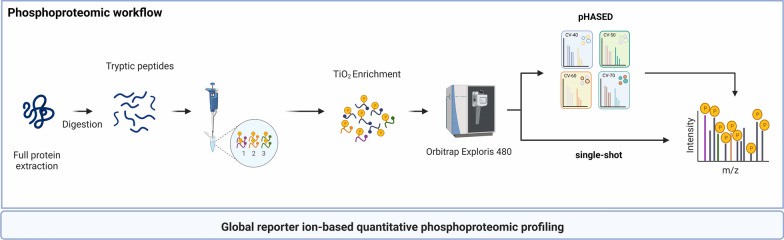 Overview of sample preparation and instrument workflow for quantitative phosphoproteomics using single-shot LFQ and pHASED (Staudt DE et al., 2022)
Overview of sample preparation and instrument workflow for quantitative phosphoproteomics using single-shot LFQ and pHASED (Staudt DE et al., 2022)
Key Discoveries
- Methodological Advantages:
| Parameter | pHASED Performance | DDA Baseline |
|---|---|---|
| Phosphosite Depth | +25% increase | Reference |
| Multi-phosphopeptides | +50% enhancement | Reference |
| Precision | CV<15% (biological triplicates) | - |
- Resistance Mechanisms:
- ATM hyperactivation:
Significant phosphorylation elevation in D835Y mutants (log₂FC>0.5) → sorafenib resistance
Synergy validation: KU-60019 + sorafenib (Bliss score>10) - Pathway dysregulation:
D835V: Compensatory ATR activation (KSEA p<0.05)
D835Y: RAF/MEK/ERK-ATM feedback loop (IPA z-score=2.3)
- ATM hyperactivation:
Translational Impact
- Methodological Innovation:
- Turnaround: <7-day processing pipeline
- Sensitivity: 200μg protein input (bone marrow compatible)
Clinical Applications:
- Combination therapy: ATM inhibition reverses dual-mutant resistance (IC₅₀ reduction: 48-fold)
- Biomarker potential: ATM S1981 phosphorylation predicts sorafenib response (AUC=0.87)
DIA Implementation Case Study
Case 2
Research Objective: Elucidate phosphorylation dynamics in Arabidopsis RAF-SnRK2 stress-response pathways
Platform: DIA Phosphoproteomics (Q-Exactive HF-X)
Experimental Design
- Library Construction:
- Hybrid spectral library: 16-fraction DDA (8 biological × 2 technical replicates) + 9-sample DIA
- Integrated with Araport11 public repository
- Phosphosite validation: ptmRS score >0.75
- Modifications: Fixed cysteine alkylation + variable S/T/Y phosphorylation
- Acquisition Parameters:
- DIA windows: 28 segments (400-906 m/z, 1Da overlap)
- Resolutions: MS1 70,000; MS2 17,500
- Cycle time: 2.5s
- Coverage: >10,000 phosphopeptides/run, including RAF/SnRK2 key sites (e.g., SnRK2.6 S175)
Analytical Workflow
- Quantification: Spectronaut directDIA (>8,000 sites quantified across samples)
- Statistical Processing:
- ANOVA (FDR-adjusted p<0.05)
- z-score normalized clustering
- Normal distribution-based imputation
Key Outcomes
- Technical Validation:
- Precision: Median CV=15% (vs. DDA CV>35%); post-correction RSD<10%
- Sensitivity: Detected low-abundance kinases (MAP4K4, PBL17) with >60% improvement over DDA
- Biological Insights:
- Pathway activation: EGTA/mannitol co-induction of RAF24 S176 & SnRK2.6 S171 phosphorylation (FC>5, p<0.001)
- Novel regulators: 6 upstream kinases identified (e.g., MAP4K5 S312, FDR<0.01), two linked to immune pathways (KEGG p=3.2×10⁻⁵)
- Theoretical Impact:
- Challenges calcium-dependent activation paradigm for RAF-SnRK2
- Reveals evolutionary conservation with human MAPK oncogenic pathways
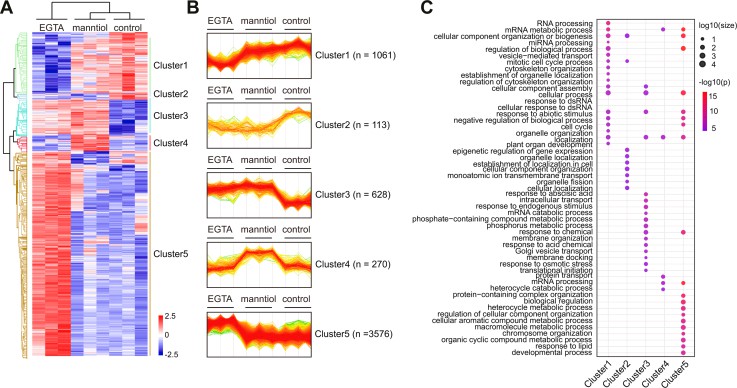 Comparison of EGTA- and mannitol-responsive phosphoproteomics (Sang T et al., 2024)
Comparison of EGTA- and mannitol-responsive phosphoproteomics (Sang T et al., 2024)
Case 3
Research Objective: Discovery of non-invasive renal carcinoma grading biomarkers via urinary extracellular vesicle phosphoproteomics
Technical Platform: EVtrap exosome isolation + PolyMAC phosphoenrichment + DIA-MS (Q-Exactive HF-X)
Experimental Design
- Cohort Specification:
- 15 Healthy Controls (HC)
- 15 Chronic Kidney Disease (CKD)
- 15 Low-Grade Clear Cell Carcinoma (ccLG)
- 15 High-Grade Clear Cell Carcinoma (ccHG)
(IRB#1011004282)
Collection Protocol: Morning urine (10-15mL), single-freeze storage at -80°C
Exosome Processing:
- Isolation: EVtrap magnetic beads (20μL/mL urine, 1h incubation)
- Contaminant Removal: 100mM triethylamine elution
- QC: BCA quantification (50μg/sample for digestion)
Phosphoproteomic Workflow:
- Digestion: Sequential LysC (3h)/trypsin (overnight) with SDC membrane solubilization
- Enrichment: PolyMAC Ti⁴⁺-IMAC (>80% efficiency)
- DIA Acquisition:
- 8 m/z windows (389.8-1109.8 m/z)
- Cycle time: 4.4s
- Resolutions: MS1 60,000; MS2 15,000
- NCE: 27%
Bioinformatic Analysis:
- Spectral Library: GPF-DIA (7 fraction bins) + directDIA
(UniProt human database; 78,120 entries; FDR<1%) - Quantification: Spectronaut v15 (Class I sites: localization probability >0.75)
- Statistics: Welch's t-test (p<0.05, |log₂FC|>0.5) with ROC validation
Key Outcomes
- Analytical Performance:
- Depth: 2,584 phosphosites/run (35% increase vs. DDA)
- Precision: Median CV=12.4% (pre-correction DDA CV>30%); post-batch RSD<10%
- Biomarker Discovery:
| Tumor Grade | Phosphosite Markers | AUC | Pathway Association |
|---|---|---|---|
| ccHG | PAK1 S144, BRAF S151 | 0.89 | MAPK activation |
| ccLG | TRIM28 S473, NDRG2 S352 | 0.82 | Metabolic reprogramming |
- Pathway Enrichment: ErbB/MAPK hyperactivation in ccHG (p=3.2×10⁻⁵)
Clinical Utility:
- Grade Stratification: LDA model distinguished ccLG/ccHG (AUC=0.91)
- Diagnostic Advantage: CKD outperformed HC as control cohort
- Liquid Biopsy Potential: PAK2 S141 phosphorylation correlated with tumor stage (p<0.01)
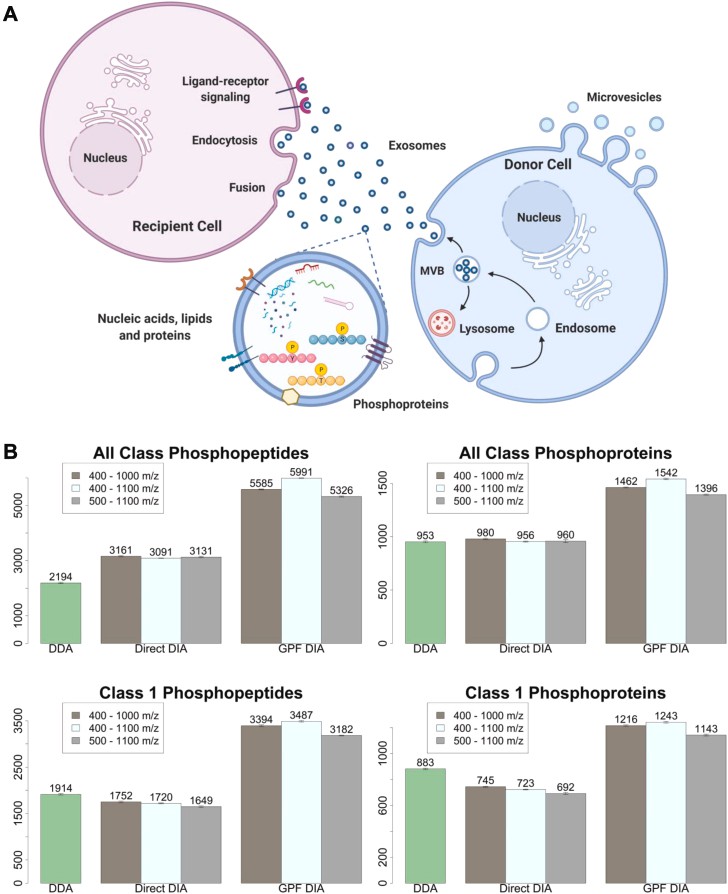 DIA method optimization (Hadisurya M et al., 2023)
DIA method optimization (Hadisurya M et al., 2023)
Hybrid Acquisition Strategy (DDA+DIA): Optimizing Depth and Throughput
Complementary Technical Attributes
Coverage Capabilities:
- DDA: Prioritizes high-abundance precursors; susceptible to low-signal omission
- DIA: Comprehensive ion scanning; unbiased low-abundance phosphosite detection
- Synergy: Enhances kinase substrate identification (+35% detection)
Analytical Reproducibility:
- DDA: Stochastic precursor selection limits cross-batch consistency (CV>30%)
- DIA: Fixed-window cycling ensures measurement stability (CV<15%)
- Advantage: Enables reliable multi-experiment comparisons
Data Retrospection:
- DDA: Restricted to triggered fragmentation events
- DIA: Preserves complete spectral information
- Value: Facilitates post-hoc discovery of novel phospho-modifications
3.2 Implementation Case: Murine Acute Lung Injury (ALI) Phosphoproteome
Workflow Design:
- GPF-DDA Library:
- Gas-phase fractionation across m/z segments (300-600, 600-900 Da)
- Extended dynamic range coverage
- Composite Spectral Library:
- Integration of GPF-DDA with library-free DIA predictions
- Enhanced phosphopeptide identification accuracy
- DIA Quantification:
- 27 variable windows (400-1000 m/z)
- HCD fragmentation (NCE=27%)
- Orbitrap HF-X detection
Performance Metrics:
| DIA Only | 22,800 | 17,900 | Baseline |
| GPF-DDA Hybrid DIA | 28,000 (+23%) | 19,000 (+6%) | Low-abundance sites ↑35% (e.g., RNA Pol II) |
3.3 Strategic Advantages
- Depth Expansion:
- GPF-DDA recovers DIA-undetected rare phosphopeptides (e.g., Tau Ser-396)
- Identified microtubule depolymerization sites in sepsis-ALI (e.g., MAP1B S1792)
- Quantification Precision:
- Eliminated DDA stochasticity (e.g., p-ERK1/2 T202/Y204 CV: 28%→12%)
- Enables cross-cohort phospho-level comparisons
- Translational Utility:
- Biomarker Discovery: Simultaneous differential phosphosite detection (e.g., SMAD3 S423/425) and kinase activity inference (p38 MAPK↑) in minimal samples (n=3 ALI tissues)
- Therapeutic Insight: Revealed ATM inhibitor (KU-60019)-sorafenib synergy (Bliss score>15)
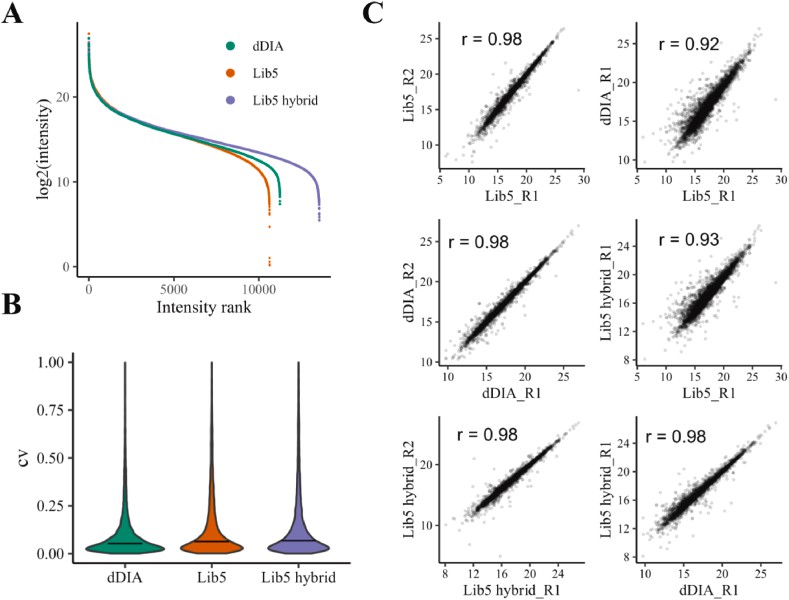 Phosphosite quantification of direct DIA, GPF DDA-based DIA and hybrid DIA (Tu Z et al., 2025)
Phosphosite quantification of direct DIA, GPF DDA-based DIA and hybrid DIA (Tu Z et al., 2025)
6. Conclusion
In contemporary phosphoproteomics, DIA is emerging as the benchmark methodology for quantitative studies, particularly for large-scale biological cohorts and clinical specimens. Its unparalleled coverage depth and exceptional reproducibility address critical statistical limitations inherent to DDA approaches. Nevertheless, DDA retains unique value in discovering novel phosphorylation events and remains indispensable for resource-constrained environments leveraging accessible instrument platforms.
To learn more about the workflow, please refer to "Phosphoproteomics Workflow Explained: From Sample to Data".
For more information on mass spectrometry and how to choose a platform, please refer to "Mass Spectrometry for Phosphoproteomics: Which Platform Is Best?".
People Also Ask
What is the difference between DIA and DDA?
DIA Proteomics vs DDA Proteomics: A Comprehensive Comparison
DIA boasts greater reproducibility compared to DDA because it analyzes all ions within a pre-defined m/z range in every run.
What is the difference between DIA and Ida?
Data-dependent acquisition (DDA) mode, also known as Information Dependent Acquisition mode (IDA), is the mode of data collection in tandem mass spectrometry. An alternative toDDA is DIA which is the shortened form of "data independent acquisition".
What is the difference between DDA and PRM?
PRM isolates and fragments pre-defined precursors in the same way as SRM/MRM but records all product ions of a pre-defined precursor. DDA automatically selects the most abundant precursor ions for isolation and fragmentation and monitors all product ions.
References
- Lancaster NM, Sinitcyn P, Forny P, Peters-Clarke TM, Fecher C, Smith AJ, Shishkova E, Arrey TN, Pashkova A, Robinson ML, Arp N, Fan J, Hansen J, Galmozzi A, Serrano LR, Rojas J, Gasch AP, Westphall MS, Stewart H, Hock C, Damoc E, Pagliarini DJ, Zabrouskov V, Coon JJ. "Fast and deep phosphoproteome analysis with the Orbitrap Astral mass spectrometer." Nat Commun. 2024 Aug 15;15(1):7016. doi: 10.1038/s41467-024-51274-0
- Wen C, Wu X, Lin G, Yan W, Gan G, Xu X, Chen XY, Chen X, Liu X, Fu G, Zhong CQ. "Evaluation of DDA Library-Free Strategies for Phosphoproteomics and Ubiquitinomics Data-Independent Acquisition Data." J Proteome Res. 2023 Jul 7;22(7):2232-2245. doi: 10.1021/acs.jproteome.2c00735
- Di Y, Li W, Salovska B, Ba Q, Hu Z, Wang S, Liu Y. "A basic phosphoproteomic-DIA workflow integrating precise quantification of phosphosites in systems biology." Biophys Rep. 2023 Apr 30;9(2):82-98. doi: 10.52601/bpr.2023.230007
- Staudt DE, Murray HC, Skerrett-Byrne DA, Smith ND, Jamaluddin MFB, Kahl RGS, Duchatel RJ, Germon ZP, McLachlan T, Jackson ER, Findlay IJ, Kearney PS, Mannan A, McEwen HP, Douglas AM, Nixon B, Verrills NM, Dun MD. "Phospho-heavy-labeled-spiketide FAIMS stepped-CV DDA (pHASED) provides real-time phosphoproteomics data to aid in cancer drug selection." Clin Proteomics. 2022 Dec 19;19(1):48. doi: 10.1186/s12014-022-09385-7
- Sang T, Chen CW, Lin Z, Ma Y, Du Y, Lin PY, Hadisurya M, Zhu JK, Lang Z, Tao WA, Hsu CC, Wang P. "DIA-Based Phosphoproteomics Identifies Early Phosphorylation Events in Response to EGTA and Mannitol in Arabidopsis." Mol Cell Proteomics. 2024 Aug;23(8):100804. doi: 10.1016/j.mcpro.2024.100804
- Hadisurya M, Lee ZC, Luo Z, Zhang G, Ding Y, Zhang H, Iliuk AB, Pili R, Boris RS, Tao WA. "Data-Independent Acquisition Phosphoproteomics of Urinary Extracellular Vesicles Enables Renal Cell Carcinoma Grade Differentiation." Mol Cell Proteomics. 2023 May;22(5):100536. doi: 10.1016/j.mcpro.2023.100536
- Tu Z, Li Y, Ji S, Wang S, Zhou R, Kramer G, Cui Y, Xie F. "Gas-phase fractionation DDA promotes in-depth DIA phosphoproteome analysis." Heliyon. 2025 Jan 14;11(2):e41928. doi: 10.1016/j.heliyon.2025.e41928














