Isoleucine is an essential branched chain amino acid (BCAA) that the human body cannot synthesize by itself and must be ingested through diet. It, together with leucine and valine, constitutes the BCAA family and plays multiple key roles in maintaining the metabolic homeostasis and health of the body. Although isoleucine plays a fundamental role in protein synthesis, recent studies have increasingly emphasized its unique status as an energy substrate and metabolic regulator, especially in the aspects of metabolic diseases and immune function regulation.
Isoleucine Metabolic Pathway
Absorption and Transport of Isoleucine
Isoleucine is absorbed in the small intestine primarily through active transport mechanisms. The Lat1 (L-type amino acid transporter 1) is one of the key transporters involved in this process. It facilitates the uptake of isoleucine from the intestinal lumen into the enterocytes. However, the absorption of isoleucine is not an isolated process. It competes with other BCAAs, such as leucine and valine, for the same transporters. This competition can influence the rate and extent to which isoleucine is absorbed and subsequently transported into the bloodstream. The competition among these amino acids for transporters like Lat1 highlights the intricate balance in the absorption dynamics of BCAAs.
Tissue Distribution and Transport Characteristics
Once absorbed, isoleucine is distributed to various tissues throughout the body. The muscle tissue is a major site of isoleucine metabolism. It has a high capacity for isoleucine uptake and utilization due to the expression of relevant metabolic enzymes. In contrast, the liver, which is typically a central organ for many metabolic processes, expresses relatively lower levels of the enzymes involved in BCAA metabolism. This unique distribution pattern means that the muscle tissue plays a predominant role in the initial steps of isoleucine catabolism, while the liver contributes to a lesser extent in this specific metabolic context.
Major Isoleucine Metabolic Pathways
Transamination
The catabolism of isoleucine begins with a transamination reaction catalyzed by branched-chain aminotransferase (BCAT), an enzyme localized in both the cytosol and mitochondria, with BCATm being the predominant isoform in peripheral tissues. BCAT facilitates the transfer of the α-amino group from isoleucine to α-ketoglutarate, yielding glutamate and α-keto-β-methylvalerate (also known as α-ketoisocaproate, α-KIC), a key BCKA intermediate. This reaction represents the first commitment step in BCAA catabolism and is reversible under physiological conditions, allowing for dynamic flux based on cellular energy status and nitrogen balance.
Oxidative Decarboxylation
Following transamination, α-KIC undergoes oxidative decarboxylation catalyzed by the multienzyme branched-chain α-keto acid dehydrogenase (BCKDH) complex, a mitochondrial matrix-resident complex structurally and functionally analogous to pyruvate and α-ketoglutarate dehydrogenase complexes. The BCKDH complex irreversibly decarboxylates α-KIC, yielding isobutyryl-CoA, while concomitantly reducing NAD⁺ to NADH and releasing CO₂. The activity of BCKDH is tightly regulated via phosphorylation by BCKDH kinase (BDK), which inactivates the complex, and dephosphorylation by BCKDH phosphatase (PPM1K), which reactivates it. This regulatory mechanism integrates signals of cellular energy demand, redox status, and amino acid availability.
Subsequent Metabolism as an Energy Substrate
Isobutyryl-CoA then undergoes a series of downstream reactions, including dehydrogenation to methylacrylyl-CoA by isobutyryl-CoA dehydrogenase (IBD), hydration to 2-hydroxy-3-methylbutyryl-CoA, and subsequent conversion to propionyl-CoA via methylmalonic acid intermediates. Propionyl-CoA is finally converted into succinyl-CoA through a three-step pathway involving propionyl-CoA carboxylase (PCC), methylmalonyl-CoA epimerase, and methylmalonyl-CoA mutase (dependent on adenosylcobalamin as a cofactor). Succinyl-CoA then enters the tricarboxylic acid (TCA) cycle, linking isoleucine catabolism directly to cellular energy production via oxidative phosphorylation.
This metabolic pathway provides both glucogenic and ketogenic outputs, as the carbon skeleton of isoleucine is partitioned between succinyl-CoA and acetyl-CoA generation. Thus, isoleucine is classified as both a glucogenic and ketogenic amino acid. Dysregulation of this pathway, such as through inherited deficiencies in BCKDH complex function (as observed in maple syrup urine disease), leads to toxic accumulation of BCKAs, with neurotoxic consequences particularly affecting the developing brain. Additionally, abnormal BCAA metabolism has been implicated in metabolic disorders such as insulin resistance, type 2 diabetes, and cancer, through mechanisms involving mTOR signaling, mitochondrial overload, and branched-chain-derived acylcarnitines.
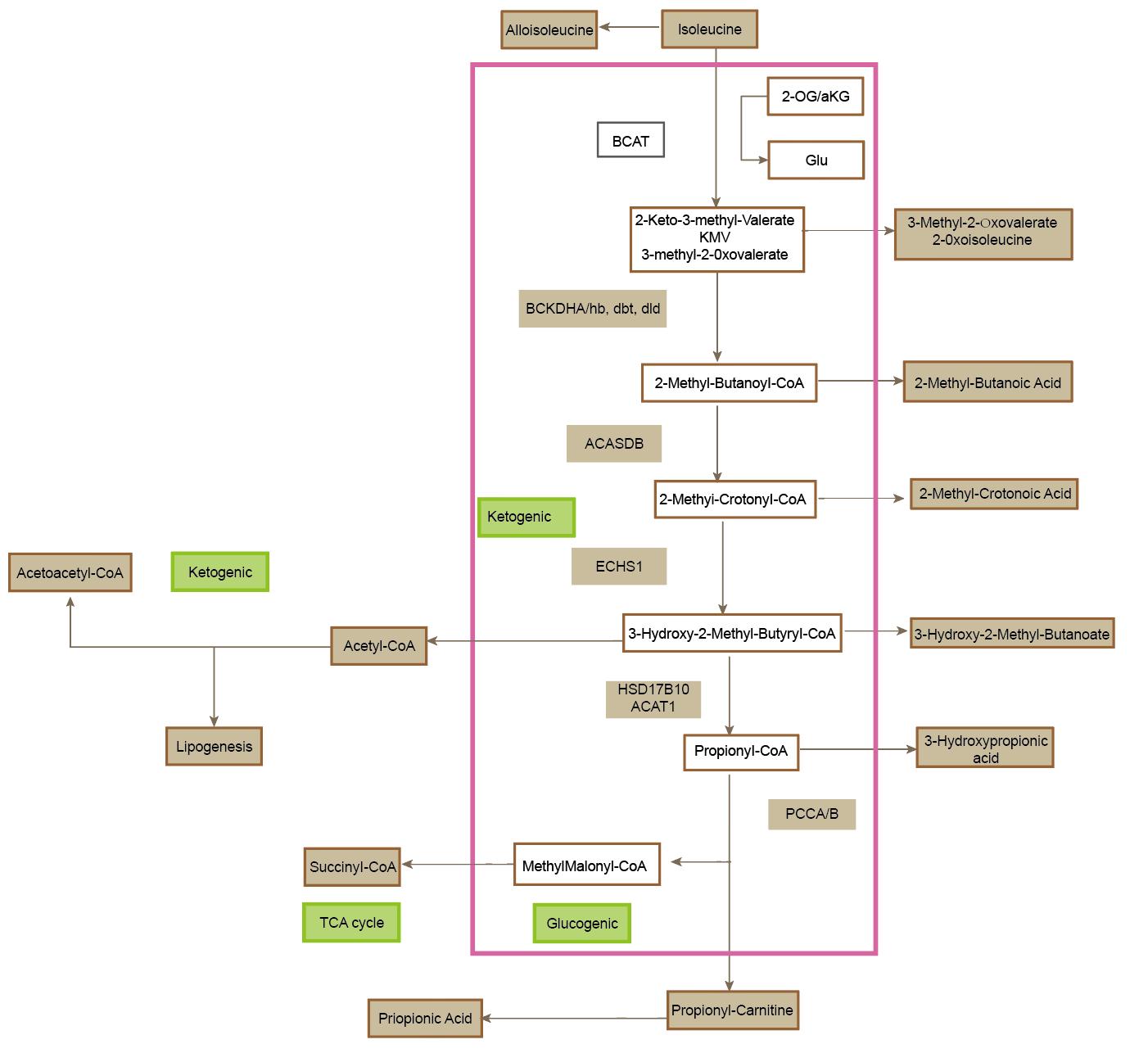 Figure 1. The metabolic pathways of isoleucine.
Figure 1. The metabolic pathways of isoleucine.
Regulation of Isoleucine Metabolism
Enzyme Regulation Mechanisms
The metabolism of isoleucine is primarily regulated at the enzymatic level, with BCAT and BCKDH complex being the key enzymes involved.
BCAT catalyzes the initial step of isoleucine metabolism, converting it to α-ketoisocaproate through a transamination reaction. The expression and activity of BCAT can be influenced by various factors, including nutritional status and hormonal signals (Zhou, F. et al. 2024). The BCKDH complex, responsible for the oxidative decarboxylation of α-ketoisocaproate to isobutyryl-CoA, is subject to intricate regulatory mechanisms. One prominent regulatory mechanism involves phosphorylation. BCKDH kinase (BCKDK) phosphorylates the E1α subunit of BCKDH (BCKDHA), thereby inhibiting its activity. Conversely, protein phosphatase PP2Cm (encoded by the gene PPM1K) dephosphorylates BCKDHA, thereby promoting its activity. This phosphorylation-dephosphorylation cycle allows for fine-tuned regulation of BCKDH activity in response to cellular needs.
Nutritional Status and Hormonal Impact
Insulin plays a crucial role in regulating the metabolism of isoleucine. Elevated levels of insulin positively regulate the activity of metabolic enzymes involved in isoleucine catabolism. Insulin promotes the uptake of isoleucine into cells and enhances the activity of BCAT and BCKDH, thereby facilitating the breakdown of isoleucine to produce energy substrates. Similarly, glucose availability also impacts isoleucine metabolism. Adequate glucose levels support the activity of these metabolic enzymes, ensuring efficient isoleucine catabolism. When glucose is abundant, cells preferentially utilize glucose for energy production, and the catabolism of isoleucine is maintained at a steady rate to provide additional energy substrates and precursors for biosynthetic processes.
Genetic and Epigenetic Regulation
Genetic mutations in the genes encoding key enzymes involved in isoleucine metabolism can lead to severe metabolic disorders. Mutations in the BCKDHA and BCKDHB genes, which encode subunits of the BCKDH complex, are associated with maple syrup urine disease (MSUD). MSUD is a rare but serious genetic disorder characterized by the accumulation of branched-chain amino acids and their corresponding α-keto acids in the blood and urine(). The name of the disease originates from the characteristic maple syrup-like odor of the affected individuals' urine. The accumulation of these metabolites is toxic and can cause severe neurological damage, leading to intellectual disability, seizures, and even death if left untreated. Early diagnosis and dietary management, including the restriction of branched-chain amino acids, are essential for managing MSUD and preventing complications.
Physiological Roles of Isoleucine Metabolism
Energy Metabolism
Isoleucine plays a significant role in energy metabolism, particularly as a substrate for the TCA cycle. When metabolized, isoleucine is converted into intermediates such as succinyl-CoA and acetyl-CoA, which can enter the TCA cycle to produce ATP. This process is particularly important during periods of increased energy demand, such as during exercise. During exercise, the oxidation of BCAAs, including isoleucine, increases in muscle tissue. This enhanced oxidation allows BCAAs to contribute a larger proportion of the total energy supply, thereby supporting prolonged physical activity.
Glucose Metabolism and Insulin Sensitivity
Isoleucine has been shown to promote the expression of GLUT4, a glucose transporter, thereby enhancing glucose uptake in muscle tissue. This effect can improve insulin sensitivity and glucose metabolism. However, elevated levels of BCAAs, including isoleucine, have also been associated with insulin resistance in some studies. The relationship between BCAA levels and insulin resistance is complex and may be influenced by factors such as obesity and genetic predisposition.
Immune Function Regulation
The metabolic support of isoleucine for immune cells
Isoleucine, as a branched-chain amino acid, is rapidly metabolized in immune cells to provide local energy support. This energy supply is crucial for the rapid proliferation and activation of immune cells. For instance, activated T cells rely on glycolysis to support their rapid proliferation, while the mTOR signaling pathway also plays a crucial role in the metabolism and function of immune cells. In addition, isoleucine may indirectly affect the function of immune cells by regulating metabolic pathways, such as glutamine synthesis.
The regulatory effect of isoleucine on T cells and NK cells
Isoleucine can regulate the functions of T cells and NK cells in multiple ways. For instance, studies have shown that isoleucine can regulate innate and adaptive immunity by inducing the expression of β -defensin. β -defensin is an important antimicrobial peptide that can enhance the host's defense ability. In addition, isoleucine may also enhance the activity of T cells and NK cells by influencing intracellular signal transduction pathways, such as the mTOR/AKT pathway.
The overall influence of isoleucine on the immune response
Isoleucine not only acts directly on immune cells, but also indirectly affects the immune response by regulating immune metabolism and cytokine networks. For example, IL-2 is a key immunomodulatory factor that can promote the proliferation and activation of T cells and NK cells. Isoleucine may enhance the immune response by regulating the IL-2 signaling pathway. In addition, isoleucine can also regulate inflammatory responses and immune tolerance by secreting cytokines such as IL-6(Saraiva, M. et al.2010).
The potential applications of isoleucine
The role of isoleucine in the regulation of immune function makes it a potential therapeutic intervention target. For example, supplementing isoleucine can enhance the body's disease resistance. Furthermore, studies on specific metabolic pathways (such as the TLR4/MyD88/NF-κB pathway) have shown that isoleucine may play a protective role in diseases such as inflammatory bowel disease.
Interaction with the Gut Microbiota
The gut microbiota can influence the availability of isoleucine and, in turn, affect metabolic health. Certain gut bacteria can metabolize isoleucine, thereby affecting its bioavailability for host metabolism. This interaction between the gut microbiota and isoleucine metabolism highlights the importance of maintaining a healthy gut microbiome for overall metabolic health.
Health Impacts of Isoleucine
Isoleucine has multiple effects on human health, and its effects vary depending on the intake and individual differences. Based on the available data, moderate intake of isoleucine may be beneficial to metabolic health, but excessive intake may have negative effects.
The Benefits of Metabolic Health
Isoleucine helps lower blood sugar levels by promoting the uptake and utilization of glucose by muscle cells. For instance, studies have shown that oral isoleucine can significantly lower fasting blood glucose levels, increase the oxidation of glucose, and simultaneously inhibit gluconeogenesis in the liver. In addition, isoleucine has been found to improve insulin sensitivity, thereby contributing to blood sugar control in diabetic patients.
In animal experiments, limiting the intake of isoleucine can improve metabolic health, including reducing adipose tissue, enhancing insulin sensitivity and increasing energy expenditure. These studies suggest that appropriately reducing the intake of isoleucine may be an effective strategy for preventing obesity and metabolic syndrome.
The Risk of Excessive Intake
However, excessive intake of isoleucine may lead to a series of health problems. For instance, high-dose intake of isoleucine is associated with an increased risk of insulin resistance, type 2 diabetes and cardiovascular diseases. In addition, excessive intake may also cause gastrointestinal discomfort, such as nausea, vomiting and diarrhea.
In some cases, excessive intake of isoleucine may even cause damage to liver and kidney functions and increase the risk of mental health problems such as depression. Therefore, although isoleucine has potential benefits in exercise recovery and muscle growth, the safety and efficacy of its supplement form still need further research.
Differences in Gender and Age
The impact of isoleucine on metabolic health also shows differences in gender and age. For instance, in mouse experiments, low levels of isoleucine intake were beneficial for both male and female mice, but in some cases, this effect was more pronounced in the males. Furthermore, younger individuals may be more likely to benefit from a diet restricted to isoleucine, while older individuals may require a higher intake of isoleucine to maintain normal metabolic functions.
Abnormal Isoleucine Metabolism and Its Treatment
Abnormal metabolism of isoleucine can lead to a variety of diseases, such as chronic malnutrition, hypoproteinemia, and hereditary metabolic disorders (e.g., isoleucinemia). Treatment strategies include dietary adjustments, amino acid supplementation, and gene therapy.
Metabolic Engineering in Industrial Production of Isoleucine
In industrial production, optimizing the biosynthetic pathway of Isoleucine through metabolic engineering is a key strategy for increasing yield. For example, overexpression of the ilvA gene, knockout of feedback suppressor genes, and strengthening of the transport system can significantly enhance the production efficiency of isoleucine. Furthermore, by combining the dynamic regulation strategy with the static regulation strategy, the yield of 4-hydroxyisoleucine can be further increased.
Overexpress the Key Enzyme genes
The biosynthetic pathway of isoleucine involves multiple key enzymes, such as aspartic acid kinase (IlvA), hyperserine dehydrogenase (IlvB), hyperserine kinase (IlvC), etc. By overexpressing the genes of these enzymes, the synthetic capacity of isoleucine can be increased. For instance, Guilloulet et al. achieved a isoleucine yield of 40 g/L by overexpressing the ilvBN, ilvC and ilvD genes in Corynebacterium glutamicum. Furthermore, studies have also shown that overexpression of enzyme genes that resist feedback inhibition (such as HD, HT, DH and AHAS) can also significantly increase the production of isoleucine.
Knockout the Feedback Suppressor gene
The synthesis of isoleucine is subject to feedback inhibition by its precursor substances, such as α -ketoglutaric acid. By knocking out the genes involved in feedback inhibition, this inhibition can be relieved, thereby improving the synthesis efficiency of isoleucine. For example, by knockout the ilvA and panBC genes, the feedback inhibition of L-isoleucine synthesis can be effectively relieved. In addition, knocking out the brnQ gene can also reduce the secretion blockade of isoleucine and further increase the yield.
Strengthen the Rransportation System
The rapid secretion of isoleucine is crucial for avoiding intracellular accumulation and maintaining enzyme activity. By enhancing the expression of transport proteins on the cell membrane, the excretion capacity of isoleucine can be improved. For example, knocking out the brnQ gene and overexpressing the brnM gene can significantly reduce the accumulation of isoleucine. In addition, overexpressing genes encoding effector transporters (such as febm) can also effectively enhance the secretion efficiency of isoleucine.
Dynamic Regulation Strategy
Dynamic regulatory strategies include adjusting metabolic flow distribution to balance cell growth and the synthesis of target products. For instance, in the production of 4-hydroxyisoleucine, by dynamically regulating the tricarboxylic acid cycle (TCA cycle), the metabolic flow rate can be redistributed, thereby enhancing the synthesis efficiency of 4-hydroxyisoleucine. Furthermore, by overexpressing genes related to NADPH supply (such as zwf and nrdP), the REDOX balance of cells can be enhanced, thereby further increasing the yield of the target product.
Static Regulation Strategy
The static regulatory strategy mainly optimizes the rate-limiting enzyme genes in the metabolic pathway through gene editing technology. For example, by up-regulating the expression of ilvA and ilvB genes, the production of isoleucine can be significantly increased. Furthermore, by down-regulating the enzyme genes related to the competitive pathway (such as odhA), resource competition can also be reduced, thereby enhancing the synthetic efficiency of the target product.
Comprehensive application
Combining dynamic and static regulatory strategies can further optimize metabolic pathways. For instance, in Corynebacterium glutamicum, by overexpressing the ilvBN, ilvC and ilvD genes and knocking out the brnQ gene, the yield of isoleucine can be significantly increased. Furthermore, in the production of 4-hydroxyisoleucine, higher yields can be achieved by dynamically regulating the TCA cycle and combining it with static regulatory strategies (such as up-regulating related enzyme genes).
References
- Zhou, F., Sheng, C., Ma, X. et al. BCKDH kinase promotes hepatic gluconeogenesis independent of BCKDHA. Cell Death Dis 15, 736 (2024). https://doi.org/10.1038/s41419-024-07071-0
- Saraiva, M., & O'Garra, A. (2010). The regulation of IL-10 production by immune cells. Nature reviews. Immunology, 10(3), 170–181. https://doi.org/10.1038/nri2711




