Phosphoproteomics is evolving beyond static characterization toward dynamic network medicine. Three transformative waves—single-cell resolution, real-time monitoring, and clinical translation—will redefine how we comprehend disease mechanisms and advance therapeutic discovery. This work examines critical technological inflection points and strategic frameworks to establish next-generation signaling network analysis.
Technological Disruption: Overcoming Existing Paradigm Limitations
1. Comprehensive Coverage Revolution: Mapping "All Sites" Beyond "Thousands"
Challenge:
Current methodologies detect <10% of potential phosphorylation sites with >10-fold dynamic range limitations, necessitating technological breakthroughs for phosphoproteome-wide studies.
Solutions:
A. Third-Generation Enrichment Materials
Engineered Nanoreceptors: Surface covalent modification of TiO₂/Ti-IMAC (e.g., with Zr⁴⁺-MOFs) significantly enhances binding energy (threefold improvement). These nanomaterials exhibit superior selectivity/affinity for broader phosphosite capture.
B. Biomimetic Phosphatase Probes
Directional capture of multiply phosphorylated peptides overcomes IMAC/TiO₂ preference bias. By emulating natural enzyme functions, these probes increase recovery efficiency for complex phosphosites missed by traditional methods.
C. Integrated Enrichment-Mass Spectrometry
Online LC-MS Enrichment: Microfluidic TiO₂ columns enable real-time enrichment, eliminating transfer losses and achieving 85% recovery. This integration enhances process efficiency, accuracy, and reduces operational errors.
D. AI-Optimized Elution
Dynamic adjustment of pH/ionic strength during elution via artificial intelligence accommodates diverse phosphorylation states. Automated optimization expands detectable phosphosite coverage and improves complex sample adaptability.
2. Spatiotemporal Resolution Leap: From Population-Level to Single-Cell Dynamics
Single-Cell Phosphoproteomics (scPhos) Methodologies
Advancements in scPhos technology now deliver enhanced resolution perspectives on intracellular signaling dynamics. Current leading methodologies enabling deep investigation of phosphorylation regulation at single-cell resolution include:
A. pSCoPE (Phosphorylation-Specific Capture and On-line Proteomic Evaluation)
- Principle: Integrates TMT labeling with phospho-specific antibody capture for efficient analysis
- Throughput/Coverage: Processes 300 cells per batch with high sensitivity
- Applications: Tumor microenvironment heterogeneity studies, comparative phosphostatus analysis across cell populations
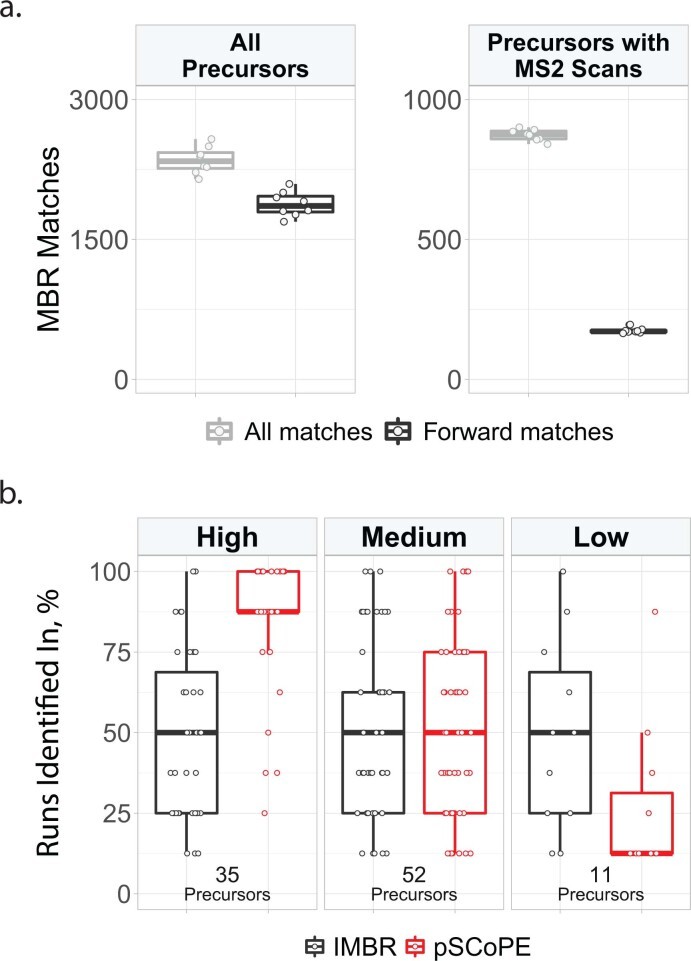 pSCoPE outperforms isobaric Match Between Runs (iMBR) for increasing consistency of identification across single-cell experiments (Huffman RG et al., 2023)
pSCoPE outperforms isobaric Match Between Runs (iMBR) for increasing consistency of identification across single-cell experiments (Huffman RG et al., 2023)
B. NanoPOTS-SWATH
- Principle: Utilizes nanodroplet processing combined with SWATH/DIA mass spectrometry for enhanced resolution
- Resolution/Coverage: Detects >50 phosphosites per cell with single-cell precision
- Applications: Neural signaling pathway research, revealing dynamic signaling alterations in neurons
C. Photon-Encoded Flow Cytometry
- Principle: Employs DNA barcoding with phosphate-specific probes for rapid population analysis
- Throughput: Analyzes 10,000 cells hourly with ultrahigh efficiency
- Applications: Large-scale immune cell activation screening (immunology/cancer research)
D. Live-Cell Dynamic Monitoring
FRET Biosensor Arrays
- Principle: Tracks >50 kinase activities simultaneously via fluorescence resonance energy transfer
- Temporal Resolution: Achieves second-scale monitoring of kinase dynamics
- Applications: Real-time signaling pathway observation, kinase activity tracking
Label-Free Raman Imaging
- Principle: Identifies phosphorylation hotspots through C-P bond Raman shifts (~980 cm⁻¹)
- Advantages: Label-free operation prevents artifactual interference, enables quantitative in situ analysis
- Applications: Non-destructive monitoring of phosphorylation dynamics during cellular differentiation
3. Data Dimension Integration: Evolving from Single-Omics to Systems Biology Engines
Modern biology increasingly requires integrated analytical approaches as single-omics methods prove insufficient for comprehensively interpreting complex biological systems. Multidimensional data integration enables researchers to decipher system-wide dynamics and mechanisms across biological hierarchies. Key cutting-edge integration platforms and innovative tools include:
Multidimensional Integration Platforms
- Phosphoproteomics: Captures protein phosphorylation modifications across cells/tissues, revealing their critical signaling roles.
- Kinase Activity Profiling: Interrogates signal transduction regulators to determine pathway activation states and temporal changes.
- Protein-Protein Interaction Networks: Maps functional cellular foundations by identifying signaling nodes and regulatory mechanisms.
- Metabolic Flux Analysis: Traces metabolite pathways to elucidate signal-mediated metabolic regulation, particularly crucial in rapidly proliferating systems like tumors.
- Dynamic Signaling-Metabolic Coupling Models: Integrates phosphoproteomic, kinase activity, interactome, and metabolic data to construct systems-level models of cellular adaptation to environmental changes.
 Signaling network analysis by mass spectrometry, drug-target discovery, and kinase inhibition (Nita-Lazar A et al., 2008)
Signaling network analysis by mass spectrometry, drug-target discovery, and kinase inhibition (Nita-Lazar A et al., 2008)
Core Innovation Tools
A. PhosFlow-CyTOF
- Principle: Combines flow cytometry with mass cytometry (CyTOF) using metal-conjugated antibodies for multiplexed phosphoprotein detection
- Function: Enables single-cell quantification of phosphoproteins with simultaneous phenotypic characterization, revealing signaling response dynamics
B. Spatial Phosphoproteomics
- Principle: Implements Digital Spatial Profiling (DSP) technology for 100μm-resolution tissue analysis
- Function: Maps spatially-resolved phosphorylation states across tissue architectures, correlating modification patterns with structural and functional contexts
Select Service
Learn more
Strategic Prioritization: Building Next-Generation Technology Ecosystems
1. Infrastructure Investment Priorities
A. Hardware Layer: High-Throughput Mass Spectrometry Imaging (MALDI-3)
- ROI Period: 3-5 years | Risk: Medium
- Capabilities: This advanced imaging technology delivers precise spatial phosphoproteomic data at cellular/tissue levels. Its breakthroughs will accelerate quantitative biomarker discovery, therapeutic development, and early disease diagnostics.
- Applications: Particularly valuable in oncology and neuroscience research. Evolving computational capabilities will enhance detection of subtle phosphorylation changes, becoming a core competitive advantage.
B. Algorithm Layer: AI-Driven Phosphosite Prediction
- ROI Period: 2-3 years | Risk: Low
- Capabilities: Machine learning algorithms significantly enhance phosphosite prediction accuracy while reducing experimental costs. These systems rapidly identify biologically significant modifications within complex datasets and infer functional impacts.
- Integration: Combined with AlphaFold2 protein modeling, AI engines predict phosphorylation-induced structural changes, improving drug target validation efficacy.
C. Data Layer: Human Phosphoproteome Knowledge Graph
- ROI Period: 5+ years | Risk: High
- Function: Integrates multi-omics data (transcriptomics, proteomics, metabolomics) to support systems biology. Serves as a critical bridge between basic research and clinical precision medicine.
- Challenges: Requires solutions for data volume, accuracy maintenance, and update frequency. Optimization needed for collection, storage, and sharing infrastructure.
2. Competitive Barrier Development
A. Patent Strategy Focus
- Synthetic Phosphosite Reference Library: Essential for analytical standardization, particularly for low-abundance unstable sites (e.g., pY peptides), enabling reliable experimental validation.
- Kinase-Substrate Interaction Database: AI-powered prediction of unknown interactions (using AlphaFold2) offers novel drug development insights and target verification pathways.
B. Standardization Initiatives
- CLIA-Certified Phosphodetection Workflows: Establishing standardized clinical detection protocols for disease-specific applications to enhance medical adoption.
- Companion Diagnostic Guidelines (FDA/EMA): Position phosphodetection as key for personalized treatment planning, advancing precision medicine through regulatory pathways.
3. Risk Mitigation: Technology Transition Pitfalls
A. Data Overload Challenges
- Issue: Single experiments generating >10⁷ data points create information redundancy and noise.
- Solution: Develop phospho-specific compression algorithms with dimensionality reduction to extract biologically relevant features while conserving computational resources.
B. Clinical Validation Barriers
- Issue: Tissue-specific phosphosite variability compromises diagnostic accuracy due to functional heterogeneity across organs.
- Solution: Establish standardized phosphopeptide reference materials to overcome batch effects and biological variability, ensuring result reproducibility.
Ethics and Regulatory Framework
1. Data Privacy Protection
GDPR Compliance Solution:
Developed blockchain-based anonymization system for phosphoproteomic data (Hyperledger Fabric architecture), enabling:
- Full traceability of data usage
- Dynamic patient authorization revocation mechanism
2. Biosafety Risk Control
Synthetic Phosphopeptide Regulation:
WHO framework-based hazard classification:
| Level | Criteria | Control Measures |
|---|---|---|
| BSL1 | <30% human homology | Standard laboratory protocols |
| BSL2 | Involving oncogenic pathways | BSC + Negative-pressure environments |
3. Clinical Submission Strategy
FDA Breakthrough Device Designation Pathway:
Accelerated approval requirements for phosphorylation detection devices:
- Required documentation:
- Prospective cohort data (n>3000)
- Multi-center reproducibility validation (CV<15%)
- Clinical utility economic analysis
Emerging Research Frontiers
Mitochondrial Phosphorylation Dark Matter
Current technologies detect <5% of mitochondrial phosphoproteins, requiring:
- Mitochondria-specific enrichment probes
- Ultra-low sample processing (<100 mitochondria)
Phosphorylation Memory Effect
Research framework for epigenetic-phosphorylation synergy:
The molecular cascade underlying the phosphorylation memory effect initiates with an external stimulus, which activates a kinase cascade. This signaling pathway leads to phospho-marking of target proteins, subsequently triggering chromatin remodeling. These epigenetic modifications establish transcriptional memory, ultimately resulting in persistent phenotypic changes that endure beyond the initial stimulus.
AI-Enabled Research
- PhosGPT: Phosphorylation mechanism prediction LLM trained on 30 million publications
- Virtual Phosphorylation Lab: End-to-end digital twin via NVIDIA Omniverse
Strategic Roadmap: Transitioning from Adoption to Leadership
A. Short-Term Tactics (1-2 years)
Implement DIA Phosphoproteomics + Single-Cell Platforms
- Objective: Enable precise phosphomodification quantification through data-independent acquisition (DIA) technology integrated with single-cell resolution, generating individualized biomarkers for accurate disease stratification and early detection.
- Actions:
- Establish multinational clinical cohorts with leading research institutions
- Curate phosphoproteomic databases across cancer, diabetes, and cardiovascular disease progression stages
- Develop infrastructure for personalized medicine data integration
Launch PROTAC-Based Degradation Platforms
- Objective: Develop novel targeted degraders for "undruggable" kinases using PROTAC technology, leveraging cellular E3 ubiquitin ligase systems for therapeutic innovation.
- Actions:
- Prioritize oncology/neurodegeneration-associated recalcitrant kinases
- Invest in collaborative platforms with pharma/academia for accelerated clinical translation
B. Medium-Term Strategy (3-5 years)
Pioneer Portable Phosphodetection Devices
- Objective: Create clinical-grade instrumentation for real-time phosphorylation monitoring, particularly chronic disease biomarkers (e.g., diabetes, cardiac conditions), enabling patient self-management and personalized treatment.
- Actions:
- Validate clinical performance of point-of-care detection systems
- Partner with medical device manufacturers for commercialization
- Integrate into preventive screening and chronic care pathways
Lead Global Spatial Phosphomapping Initiatives
- Objective: Establish comprehensive cellular/tissue phosphoatlases through international consortia (HCA/HuBMAP), elucidating spatially-resolved signaling mechanisms.
- Actions:
- Found multinational phosphoproteomics research alliance
- Disseminate standardized spatial mapping methodologies
- Drive academic-industrial data sharing frameworks
C. Long-Term Ecosystem (5+ years)
Develop Phospho-Centric Digital Twins
- Objective: Construct multiscale predictive models simulating disease trajectories from phosphoproteomic, clinical, and individual data to guide precision interventions.
- Actions:
- Integrate AI with longitudinal phosphoproteomic big data
- Validate models in oncology/neurodegenerative contexts
- Implement clinical decision-support applications
Advance CRISPR-Phospho Editing Therapeutics
- Objective: Engineer precision correction of pathogenic phosphorylation aberrations using CRISPR-based editors, creating transformative therapies for genetic disorders and cancers.
- Actions:
- Design site-specific phospho-editing tools
- Conduct translational studies through clinical trials
- Establish regulatory pathways for phospho-targeted gene therapies
Deeply analyzing phosphorylation networks has transitioned from a technical hurdle to a critical infrastructural race within biomedicine. Entities capable of dynamically deciphering signaling pathways will lead across the entire value chain, from target identification to personalized therapies. Over the coming decade, "phosphorylated intelligence" is poised to emerge as the fundamental benchmark for evaluating biomedical advancement.
How to choose between DIA and DDA, please refer to "DIA vs DDA in Phosphoproteomics: Which One Should You Use?".
References
- Muneer G, Chen CS, Chen YJ. "Advancements in Global Phosphoproteomics Profiling: Overcoming Challenges in Sensitivity and Quantification." Proteomics. 2025 Jan;25(1-2):e202400087. doi: 10.1002/pmic.202400087
- Huffman RG, Leduc A, Wichmann C, Di Gioia M, Borriello F, Specht H, Derks J, Khan S, Khoury L, Emmott E, Petelski AA, Perlman DH, Cox J, Zanoni I, Slavov N. "Prioritized mass spectrometry increases the depth, sensitivity and data completeness of single-cell proteomics." Nat Methods. 2023 May;20(5):714-722. doi: 10.1038/s41592-023-01830-1
- Nita-Lazar A, Saito-Benz H, White FM. "Quantitative phosphoproteomics by mass spectrometry: past, present, and future." Proteomics. 2008 Nov;8(21):4433-43. doi: 10.1002/pmic.200800231














