1. Fundamental Structural Characteristics
2. Structural Determinants of Biological Function
3. Primary Origins: Dietary Intake and Endogenous Synthesis
4. Essential Physiological Roles: Four Fundamental Functions
5. Laboratory Analytical Approaches: Core Methodologies
7. Experimental Design FAQ
8. Enhanced Data Interpretation: Fatty Acid Metrics
9. Recommended Free Databases and Tools
10. Expanded Beginner Research Framework
People Also Ask
Long-chain fatty acids (LCFAs) represent a significant subclass of fatty acids with vital biological functions, abundantly present in both dietary sources and human physiology. Grasping fundamental LCFA concepts provides essential foundational knowledge for studying lipid biology, metabolic processes, and associated pathologies. This section delivers a concise overview of LCFA characteristics, encompassing their definition, structural categorization, dietary origins, and physiological roles within living organisms.
1. Fundamental Structural Characteristics
Long-chain fatty acids (LCFAs) = Hydrocarbon backbone + Terminal carboxyl group (-COOH)
Defining Features:
- Chain Length: 14–24 carbon atoms (distinct from medium-chain [C6–12] and short-chain [<C6] fatty acids)
Saturation Classification:
1.1 Saturated Fatty Acids (SFAs)
Characterized by the absence of double bonds within the hydrocarbon chain, resulting in complete hydrogen saturation. Strong intermolecular forces confer solid-state properties at ambient temperature. Common long-chain SFAs include:
- Palmitic acid (C16:0): Predominantly found in animal fats and plant-derived oils.
- Stearic acid (C18:0): Present in animal lipids and cocoa butter, exhibiting neutral physiological effects.
1.2 Unsaturated Fatty Acids (UFAs)
Contain ≥1 double bond(s) introducing structural kinks that weaken intermolecular associations, typically maintaining liquid phase at room temperature. Categorized by bond count:
1.2.1 Monounsaturated Fatty Acids (MUFAs)
Feature a single double bond. Abundant in plant oils, nuts, and marine lipids. Demonstrated cardioprotective effects through LDL cholesterol modulation.
- Oleic acid (C18:1 ω-9): Primary dietary MUFA in olive oil, avocados, and nuts.
1.2.2 Polyunsaturated Fatty Acids (PUFAs)
Contain ≥2 double bonds. Essential nutrients requiring dietary intake. Key subclasses:
- Omega-3 Fatty Acids: Includes eicosapentaenoic acid (EPA, C20:5 ω-3) and docosahexaenoic acid (DHA, C22:6 ω-3). Sourced from fatty fish, flaxseeds, and chia seeds. Contribute to reduced inflammation, enhanced cardiovascular function, and neurological health.
- Omega-6 Fatty Acids: Exemplified by linoleic acid (C18:2 ω-6) from corn/sunflower oils. Moderate consumption supports health, though excessive intake may promote inflammatory pathways.
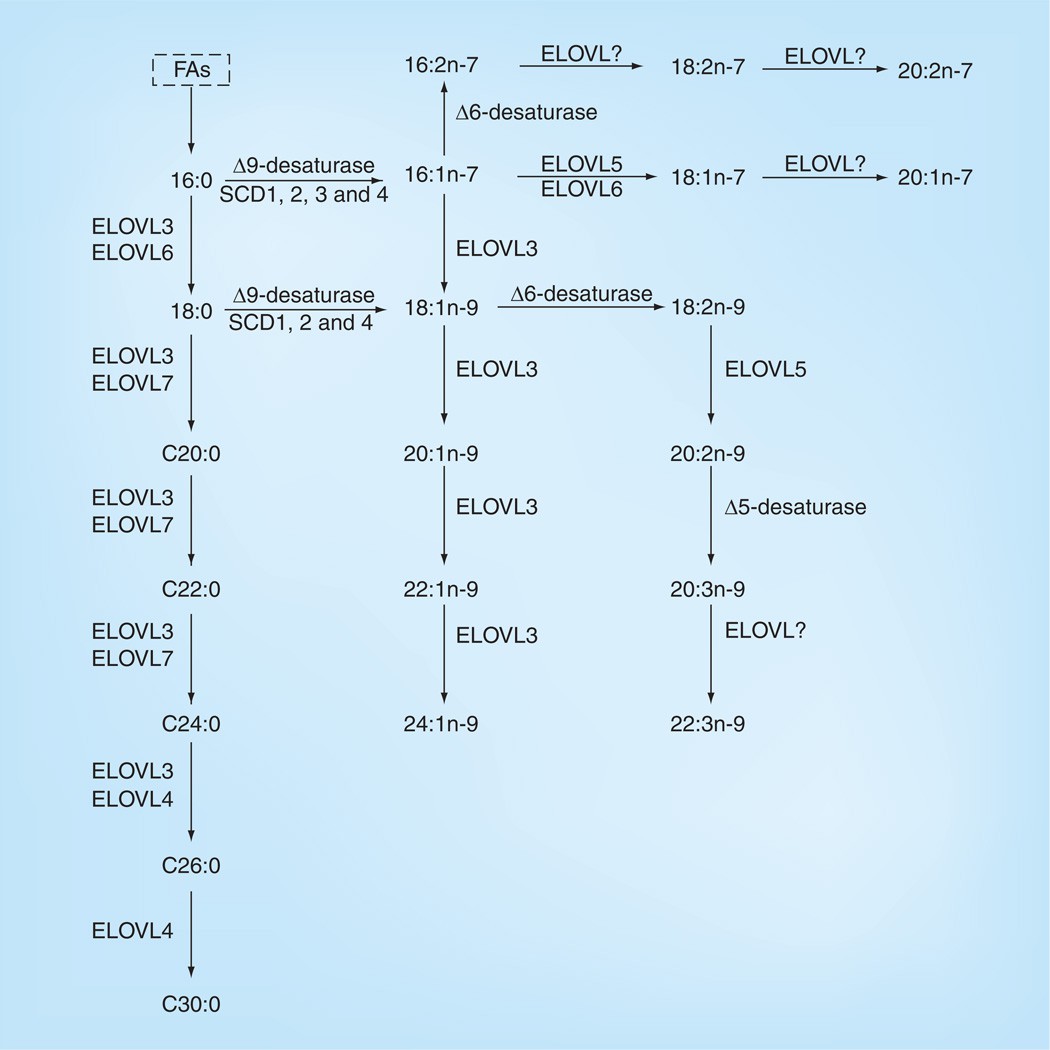 Biosynthesis of long-chain and very-long-chain saturated fatty acids and some long-chain polyunsaturated fatty acids in mammals (Liu A et al.,2011)
Biosynthesis of long-chain and very-long-chain saturated fatty acids and some long-chain polyunsaturated fatty acids in mammals (Liu A et al.,2011)
For more on the difference between long-chain fatty acids and short-chain fatty acid, see "Long-Chain vs. Medium- and Short-Chain Fatty Acids: What's the Difference?".
2. Structural Determinants of Biological Function
2.1 Carbon Chain Length
Fatty acid properties are governed by hydrocarbon chain length, which dictates melting point and fluidity. Extended chains in LCFAs strengthen van der Waals interactions, elevating melting points and typically yielding solids. Conversely, short-chain variants demonstrate greater fluidity and liquid states. Illustrative examples:
- Stearic acid (C18:0): Long-chain SFA, solid at ambient temperature
- Butyric acid (C4:0): Short-chain fatty acid from bacterial fermentation, liquid state
2.2 Double Bond Quantity
Molecular mobility increases with double bond count due to structural kinks that weaken intermolecular packing. Polyunsaturated fatty acids (PUFAs) consequently exhibit liquid characteristics at standard conditions:
- *EPA (C20:5 ω-3)* and *DHA (C22:6 ω-3)*: Highly fluid ω-3 PUFAs abundant in fish oils
- *Linoleic acid (C18:2 ω-6)*: Liquid PUFA with two double bonds
2.3 Double Bond Position
The location of the initial double bond relative to the methyl terminus (ω-carbon) defines fatty acid classification (ω-3/6/9) and corresponding physiological roles:
- ω-3 Fatty Acids (α-linolenic acid, EPA, DHA): Support cardiovascular integrity, anti-inflammatory responses, and neural function
- ω-6 Fatty Acids (e.g., linoleic acid): Modulate immune activity and cellular proliferation; excessive consumption may provoke inflammation
- ω-9 Fatty Acids (e.g., oleic acid): Cardioprotective agents prevalent in olive oil and nuts
2.4 Cis-Trans Isomerism
Geometric isomerism critically influences biological impact:
- Cis-configuration: Natural conformation enhancing membrane fluidity and cellular health
- Trans-configuration: Industrially produced variant associated with cardiovascular risks through:
- Elevating LDL cholesterol levels
- Suppressing HDL cholesterol concentrations
- Increasing incidence of cardiovascular disease and metabolic disorders
- Exemplar: Trans oleic acid in margarine and processed foods poses chronic health hazards
- Functional Summary:
| Structural Feature | Biophysical Effect | Physiological Consequence |
| Chain length | Modulates melting point & fluidity | Determines physical state (solid/liquid) |
| Double bond count | Increases molecular kinking | Enhances membrane fluidity |
| ω-position | Defines metabolic pathway | Dictates inflammatory/regulatory functions |
Illustrative Case:
Arachidonic acid (C20:4 ω-6): This 20-carbon PUFA with four double bonds serves as a crucial signaling precursor in animal tissues, modulating inflammatory responses and cellular communication pathways.
To learn more about the role of long-chain fatty acids in human health, please refer to "The Role of Long-Chain Fatty Acids in Human Health and Disease".
3. Primary Origins: Dietary Intake and Endogenous Synthesis
3.1 Dietary Sources (Primary Human Acquisition Pathway)
Long-chain fatty acids (LCFAs) are acquired both through dietary intake and endogenous biosynthesis. Diverse food sources supply distinct fatty acid profiles that support physiological functions and health maintenance:
- Plant Oils: Rich in oleic acid (olive oil) and linoleic acid (sunflower oil). Oleic acid (MUFA) reduces LDL cholesterol, while linoleic acid (ω-6) provides antioxidant and anti-inflammatory benefits, collectively lowering cardiovascular risk.
- Fatty Fish: Primary source of EPA/DHA (ω-3 PUFAs in salmon/sardines). These compounds mitigate inflammation, support neurocognitive function, and demonstrate cardioprotective effects.
- Nuts and Seeds: Contain α-linolenic acid (chia seeds, walnuts), an ω-3 precursor that undergoes conversion to EPA/DHA, exhibiting anti-inflammatory and lipid-lowering activities.
- Animal Fats: Provide palmitic acid (red meat) and stearic acid (butter). While SFAs may promote inflammation when consumed excessively, moderate intake remains compatible with cardiovascular health.
3.2 Endogenous Biosynthesis
LCFAs are synthesized intracellularly using acetyl-CoA—a carbohydrate metabolism derivative—as the foundational substrate:
- Core Substrate: Acetyl-CoA
Generated from glycolytic pathways, this metabolite serves as the primary carbon source for fatty acid assembly.
- Catalytic Machinery: Fatty Acid Synthase (FASN)
This enzyme complex catalyzes the stepwise elongation of acetyl-CoA into saturated long-chain fatty acids.
Critical Biochemical Constraint:
Humans lack Δ-12 and Δ-15 desaturases, rendering ω-3 and ω-6 PUFAs essential fatty acids. These must be obtained dietarily to maintain:
- Eicosanoid production
- Membrane fluidity
- Inflammatory regulation
Thus, adequate dietary ω-3/ω-6 intake remains physiologically indispensable.
4. Essential Physiological Roles: Four Fundamental Functions
Long-chain fatty acids (LCFAs) exhibit fundamental significance in human physiology, serving as both critical energy substrates and key regulators of cellular architecture, signaling cascades, and genomic regulation. Their core biological functions are analyzed below:
4.1 Energy Reservoir
LCFAs constitute the primary energy reserve system, with exceptional metabolic efficiency:
- Storage Mechanism: Deposited as triglycerides (TG) in adipocytes - triacylglycerol molecules comprising glycerol esterified to three fatty acid chains
- Metabolic Advantage: High energy density (9 kcal/g), doubling carbohydrate yield (4 kcal/g), making LCFAs the dominant fuel source during prolonged fasting or endurance activities
4.2 Cellular Membrane Architecture
LCFAs determine membrane structural integrity and functionality:
- Phospholipid Integration: Form hydrophobic tails of amphipathic phospholipids (e.g., phosphatidylcholine), creating the foundational bilayer
- Fluidity Modulation: DHA (docosahexaenoic acid, C22:6 ω-3) enhances neuronal membrane fluidity, optimizing synaptic transmission
- Stabilization Mechanism: Cholesterol-saturated fatty acid complexes regulate membrane microviscosity, preserving functionality across physiological conditions
4.3 Signaling Mediators
LCFA-derived molecules orchestrate intercellular communication:
- Eicosanoid Precursors: Metabolized to prostaglandins/leukotrienes governing inflammation, immunity, and vascular tone
- Nuclear Receptor Activation: PPARγ agonism by fatty acids modulates glucose homeostasis, lipid storage, and insulin sensitivity through transcriptional regulation
4.4 Genomic Regulation
Polyunsaturated LCFAs (PUFAs) exert epigenetic influences:
- Lipogenic Suppression: Downregulate sterol regulatory element-binding protein 1c (SREBP-1c), inhibiting de novo lipogenesis
- Anti-inflammatory Action: ω-3 PUFAs (EPA/DHA) attenuate NF-κB activation - the master transcriptional regulator of pro-inflammatory cytokines - reducing chronic inflammatory disease risk
5. Laboratory Analytical Approaches: Core Methodologies
Three principal techniques enable fatty acid characterization in research settings:
5.1 Gas Chromatography (GC)
- Principle: Volatilizes methyl-esterified fatty acids through thermal vaporization, separating components via gaseous phase partitioning with subsequent detector-based quantitation and structural characterization.
- Applications: Profiling total fatty acid composition in biological matrices (e.g., plasma, tissues), particularly for chain-length and saturation analysis.
- Technical Note: Requires fatty acid methyl ester (FAME) derivatization—a technically demanding but high-precision workflow for comprehensive molecular profiling.
5.2 Liquid Chromatography-Mass Spectrometry (LC-MS)
- Principle: Separates compounds through differential polarity-based interactions between stationary/mobile phases, coupled with mass detection for isomer discrimination and absolute quantitation.
- Applications: Resolving complex fatty acid mixtures in intricate samples (e.g., neural tissue), enabling cis-trans isomer differentiation and low-abundance species detection.
- Technical Note: Superior for structural isomer analysis and complex biological matrices, though requires advanced instrumentation expertise.
5.3 Enzymatic Assays
- Principle: Lipase-mediated hydrolysis of ester bonds followed by chromogenic detection of liberated products (e.g., via acyl-CoA oxidase/peroxidase reactions).
- Applications: High-throughput clinical screening of serum free fatty acids and triglycerides in diagnostic lipid panels.
- Technical Note: Rapid and accessible for beginners, but exhibits limited isomer discrimination capability and lower resolution than chromatographic techniques.
Experimental Progression Guide:
- Novice Startpoint: Initiate with enzymatic triglyceride assays for serum fatty acid level assessment
- Advanced Technique: Progress to GC-MS for detailed structural analysis and comprehensive metabolic profiling
For more information on long-chain fatty acid analysis strategies, please refer to "Analytical Strategies for Long-Chain Fatty Acids Profiling".
Services You May Be Interested In:
6. Research Significance: Implications for Health and Disease Pathogenesis
LCFAs exert pivotal influences across multiple disease pathways through distinct biological mechanisms:
Cardiovascular Health
Omega-3 LCFAs demonstrate cardioprotective efficacy, reducing serum triglycerides by approximately 30%. Conversely, excessive omega-6 intake potentiates pro-inflammatory cascades that accelerate atherosclerosis development.
Metabolic Disorders
Saturated LCFAs like palmitic acid promote insulin resistance through ceramide-mediated signaling, while monounsaturated oleic acid enhances pancreatic beta-cell functionality and glucose homeostasis.
Neurological Integrity
Docosahexaenoic acid (DHA) constitutes >25% of gray matter fatty acids, with clinically significant depletion observed in Alzheimer's disease patients, correlating with cognitive decline progression.
Emerging Research Frontiers
Gut microbiota-mediated LCFA metabolism generates immunomodulatory metabolites (e.g., butyrate), establishing novel microbiome-lipid-immune axis interactions relevant to inflammatory disorders.
7. Experimental Design FAQ
Q1: Selection Criteria: Free vs. Esterified Fatty Acid Analysis
Free Fatty Acids (FFA)
- Method: Dole extraction followed by direct GC detection.
- Purpose: Primarily employed in research on metabolic disorders including insulin resistance, with a focus on plasma FFAs. Despite comprising merely 0.5% of total plasma fatty acids, FFAs exhibit the highest metabolic activity and exert the most pronounced impact on pathological states.
Total Fatty Acids (Esterified Form)
- Method: Folch extraction of total fatty acids → methyl ester derivatization.
- Purpose: Essential for profiling fatty acid composition in tissues, particularly when examining adipose tissue or visceral fat stores where esterified forms predominate as stable reservoirs.
Specific Lipid-Bound Fatty Acids
- Method: TLC separation of phospholipids/triglycerides → methyl esterification.
- Purpose: Critical for investigating membrane lipids (e.g., phosphatidylethanolamine), where fatty acids fulfill specialized roles in structural integrity and signaling pathways.
Q2: Cellular Fatty Acid Delivery Protocol
Cellular experiments typically utilize fatty acid-albumin complexes for delivery. Albumin conjugation enables efficient transmembrane transport while mitigating direct cytotoxicity. Standard protocol:
- Dissolve palmitic acid in NaOH; clarify at 70°C.
- Incubate with 10% fatty acid-free BSA (55°C, 30 min) to ensure complete binding.
- Final concentration: 200μM palmitate with 1% BSA.
This approach ensures stable, non-toxic cellular uptake for mechanistic studies.
8. Enhanced Data Interpretation: Fatty Acid Metrics
Fatty Acid Nomenclature and Clinical Relevance
Delta (δ) Numbering System
This structural classification denotes double bond positions relative to the carboxyl terminus. Example: 18:2Δ9,12 indicates an 18-carbon chain with double bonds at carbons 9 and 12. This system facilitates rapid structural analysis linking molecular configuration to biological activity.
Omega (ω) Numbering System
Classification originates from the methyl terminus (distal to carboxyl). Example: Linoleic acid (18:2ω6) contains 18 carbons with its initial double bond at the sixth carbon from the methyl end. This method is particularly valuable for characterizing polyunsaturated fatty acids (PUFAs), especially ω-3 and ω-6 families.
Health Prognostic Indices
*ω-6/ω-3 Ratio*
- Formula: (Linoleic acid + AA) / (α-linolenic acid + EPA + DHA)
- Clinical Relevance: As essential nutrients, ω-6 fatty acids (e.g., linoleic acid) promote pro-inflammatory responses, while ω-3 compounds (EPA/DHA) exert anti-inflammatory effects. Modern diets frequently exhibit excessive ω-6 intake; maintaining a ratio below 4:1 reduces chronic inflammation and cardiovascular risks. Ratios exceeding 10:1 may potentiate inflammatory pathologies.
Saturation Index (SI)
- Formula: SFA / (MUFA + PUFA)
- Clinical Relevance: This metric predicts cardiovascular disease susceptibility. Elevated SI (>1.2) correlates with increased cardiovascular risk, as excessive saturated fatty acid (SFA) consumption may accelerate atherosclerosis and hypertension.
Thrombogenic Index (TI)
- Formula: (C14:0 + C16:0 + C18:0) / (MUFA + ω-3 PUFAs)
- Clinical Relevance: TI quantifies thrombosis propensity. Higher values indicate greater thrombotic risk, particularly with elevated dietary intake of myristic (C14:0), palmitic (C16:0), and stearic (C18:0) acids. Maintaining balanced fatty acid proportions is clinically imperative.
9. Recommended Free Databases and Tools
| Resource Name | Website | Functional Highlights |
| LIPID MAPS | lipidmaps.org | Fatty acid structure downloads + mass spectrometry data |
| Fatty Acid Explorer | go.drugbank.com/fatty_acids | Drug-fatty acid interaction queries |
| FASTAR | fastar.org | GC/MS automatic peak recognition tool |
| KEGG Fatty Acid Metabolism | kegg.jp/pathway/map00071 | Metabolic pathway visualization |
10. Expanded Beginner Research Framework
1. Replicate Foundational Experimental Protocols
Template Approach
Novice researchers should initiate studies by reproducing established experimental designs. This methodology facilitates mastery of core techniques while building technical proficiency through systematic practice.
Exemplar Protocol
- Sample Processing: 100mg liver tissue extraction for fatty acid profiling
- Methodology: Lipid extraction (Folch) → methyl ester derivatization (BF₃/methanol) → GC-MS compositional analysis
- Key Analytes: Palmitic (C16:0), oleic (C18:1), and arachidonic (C20:4) acids as fundamental biomarkers
Learning Objectives
- Comprehend GC-MS analytical principles
- Master chemical derivatization techniques for analyte detection
- Validate results against literature standards to ensure methodological robustness
2. Curate Custom Fatty Acid Database
Implementation Strategy
Develop a reference repository documenting characteristic parameters of common fatty acids to enhance experimental efficiency and data interpretation.
Critical Data Fields
- GC retention indices for compound identification
- LC-MS mass-to-charge ratios for qualitative analysis
- Diagnostic fragment ions for structural confirmation and quantitation
Optimization Guidelines
- Utilize Excel or database management systems for dynamic updating
- Cross-validate entries against established resources (e.g., USP Fatty Acid Database)
- Continuously expand repository content with experimental progression
3. Embrace Interdisciplinary Integration
Cross-Domain Synergies
Fatty acid metabolism interfaces with microbiology, environmental science, and synthetic biology, enabling novel research trajectories.
Microbiome-Metabolome Nexus
Gut microbiota enzymatically convert PUFAs into bioactive metabolites via reductase activity, significantly influencing host immunometabolism. Investigating these transformations elucidates microbiome-host crosstalk, informing dietary and probiotic interventions.
Synthetic Biology Applications
Metabolic pathway engineering in microbial systems (e.g., yeast) enables sustainable ω-3 production, offering ecologically sound alternatives to marine-sourced oils while mitigating overfishing impacts.
Core Research Philosophy
Recognize fatty acids as dynamic signaling entities rather than static molecular pools. Continuously interrogate: "How do compositional alterations modulate cellular function?" This paradigm drives mechanistic discovery and translational innovation.
Advanced Development Strategies
- Computational Proficiency: Employ statistical tools (R/Python/MATLAB) for metabolic dynamics modeling
- Literature Integration: Systematically track emerging methodologies (metabolomics/transcriptomics) in fatty acid research
- Collaborative Networks: Partner with bioinformaticians and microbiologists to expand research dimensionality
People Also Ask
What foods are high in very long chain fatty acids?
Peanuts, macadamia nuts and canola oil have the most total VLSFA, with peanuts highest in 22 : 0 and 24 : 0 and canola oil highest in 20 : 0. Among the main commercial oils, sunflower oil has noticeably more 22 : 0 [2,3]. Other commercial oils, including corn, olive, soy, safflower, contain low amounts of 20 : 0.
Is omega-6 a long chain fatty acid?
Omega-6 fatty acids are typically long-chain polyunsaturated fatty acids. The most common omega-6 fatty acid is linoleic acid, which is a 18-carbon long-chain fatty acid. Some omega-6 fatty acids, like arachidonic acid, are even longer, with 20 carbons. These fatty acids play important roles in the body, including inflammation regulation and cell membrane structure.
References
- Nakamura MT, Yudell BE, Loor JJ. "Regulation of energy metabolism by long-chain fatty acids." Prog Lipid Res. 2014 Jan;53:124-44. doi: 10.1016/j.plipres.2013.12.001
- He Q, Chen Y, Wang Z, He H, Yu P. "Cellular Uptake, Metabolism and Sensing of Long-Chain Fatty Acids." Front Biosci (Landmark Ed). 2023 Jan 16;28(1):10. doi: 10.31083/j.fbl2801010
- Kyselová L, Vítová M, Řezanka T. "Very long chain fatty acids." Prog Lipid Res. 2022 Jul;87:101180. doi: 10.1016/j.plipres.2022.101180
- Liu A, Lin Y, Terry R, Nelson K, Bernstein PS. "Role of long-chain and very-long-chain polyunsaturated fatty acids in macular degenerations and dystrophies." Clin Lipidol. 2011;6(5):593-613. doi: 10.2217/clp.11.41
- Kuo RC, Zhang H, Stuart JD, Provatas AA, Hannick L, Lin S. "Abundant synthesis of long-chain polyunsaturated fatty acids in Eutreptiella sp. (Euglenozoa) revealed by chromatographic and transcriptomic analyses." J Phycol. 2021 Apr;57(2):577-591. doi: 10.1111/jpy.13105


