Long-chain fatty acids (LCFAs, C14-C22) constitute fundamental structural components within cellular membranes while serving essential roles in energy metabolism and signaling pathways. Precise quantification of these biomolecules holds critical importance for metabolic disorder investigations, nutritional profiling, and bioenergy applications. This work comprehensively evaluates analytical methodologies spanning conventional chromatographic techniques to emerging spatial omics imaging platforms.
Comprehensive Analysis Challenges of Long-Chain Fatty Acids
Long-chain fatty acids (LCFAs, C14-C22+) serve diverse physiological roles including energy provision, membrane biogenesis, and signal transduction. Analytical characterization encounters significant obstacles due to:
Structural Complexity
- Variable chain lengths (C14 to >C22)
- Unsaturation patterns (ω-3/6/9 positioning)
- Geometric isomerism (cis/trans configurations)
- Post-synthetic modifications (hydroxylation/epoxidation)
Sample Heterogeneity
- Coexisting lipid interference (TAG/PL/CE conjugates)
- Extreme concentration ranges (millimolar plasma C16:0 to femtomolar oxidative metabolites)
Analytical Requirement Specifications
| Analysis Type | Primary Challenge | Key Applications |
|---|---|---|
| Qualitative Profiling | Structural isomer discrimination | Novel FA discovery, food authentication |
| Absolute Quantitation | Trace analyte detection (e.g., C20:4) | Biomarker validation, diagnostics |
| Metabolic Flux Tracking | Dynamic isotope tracing (¹³C-palmitate) | Pathway mapping, drug mechanism studies |
Emerging Analytical Frontiers
- Single-cell resolution: Deciphering metabolic heterogeneity (e.g., tumor microenvironment reprogramming)
- Spatial mapping: Tissue distribution profiling (e.g., ω-6 enrichment in atherosclerotic plaques)
- Real-time monitoring: Live-cell metabolic flux analysis (microfluidics-coupled biosensing)
Comparative Analysis of Core Platforms
| Method | Sensitivity (LOD) | Dynamic Range | Throughput | Typical Application Scenarios |
|---|---|---|---|---|
| GC-FID | 0.1-1 μg/mL | 10³ | Medium | Food/feed composition analysis |
| GC-MS (EI) | 0.01 μg/mL | 10⁴ | Medium-High | Comprehensive screening in biological samples |
| LC-MS/MS (MRM) | 0.001 μg/mL | 10⁵ | High | Clinical biomarker quantification |
| MALDI-TOF IMS | 10 μm/pixel | 10² | Low | Tissue spatial distribution imaging |
| Microfluidic CE | 0.1 μg/mL | 10³ | Ultra-High | Single-cell fatty acid profiling |
Analytical Platforms for Fatty Acid Profiling
1. GC-FID (Gas Chromatography-Flame Ionization Detection)
Principle: Utilizes gas chromatographic separation with flame ionization detection (FID), where ionized analytes generate quantifiable current signals.
Advantages:
- High sensitivity for low-concentration analytes
- Operational simplicity without derivatization
- Instrumental stability for high-throughput analysis
Limitations:
- Limited resolution versus MS detection
- Purely quantitative (no structural identification)
Applications: Food/feed ingredient quantification (e.g., vegetable oil fatty acid profiles)
2. GC-MS EI (Gas Chromatography-Electron Impact Mass Spectrometry)
Principle: Combines chromatographic separation with electron impact ionization and mass analysis.
Advantages:
- Enhanced sensitivity and resolution
- Structural elucidation capabilities
Limitations:
- Requires fatty acid derivatization
- Extended analysis duration
Applications: Comprehensive biological screening (e.g., serum/tissue fatty acid profiling)
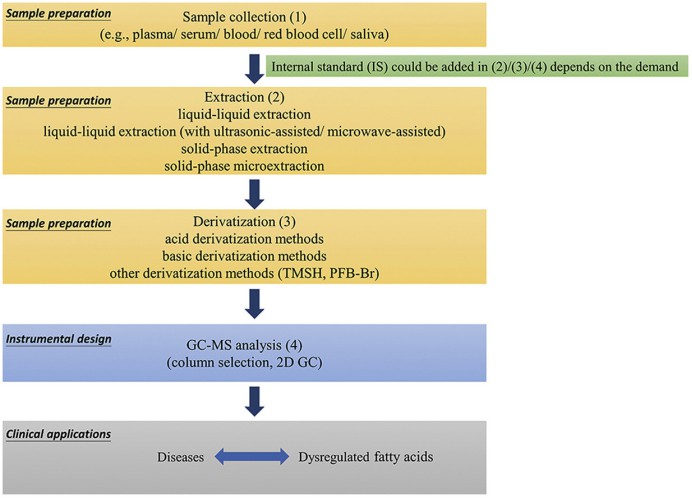 Gas chromatography-mass spectrometry-based analytical strategies for fatty acid analysis in biological samples
Gas chromatography-mass spectrometry-based analytical strategies for fatty acid analysis in biological samples
3. LC-MS/MS MRM (Liquid Chromatography-Tandem Mass Spectrometry)
Principle: Liquid chromatographic separation with multiple reaction monitoring for targeted quantification.
Advantages:
- Exceptional sensitivity (sub-ng/mL detection)
- High selectivity against matrix interference
- Rapid analysis cycles
Limitations:
- Significant capital/maintenance costs
- Requires specialized operator expertise
Applications: Clinical biomarker validation (e.g., blood/urine diagnostics)
4. MALDI-TOF IMS (Matrix-Assisted Laser Desorption/Ionization Imaging Mass Spectrometry)
Principle: Laser desorption/ionization with spatial resolution for molecular imaging.
Advantages:
- Spatial distribution mapping
- Non-destructive sample analysis
Limitations:
- Low throughput capacity
- Complex sample preparation
Applications: Tissue spatial analysis (e.g., tumor microenvironment studies)
5. Microfluidic Capillary Electrophoresis
Principle: Electrophoretic separation in microfabricated channels.
Advantages:
- Ultra-high throughput processing
- Single-cell resolution capability
Limitations:
- Restricted concentration range optimization
- Technically demanding fluidic control
Applications: Single-cell metabolomics (e.g., cellular heterogeneity studies)
To learn how to choose the right LC-MS platform, please refer to "Choosing the Right LC-MS Platform for Fatty Acid Analysis".
Services You May Be Interested In:
Pretreatment Method Selection for LCFA Profiling
Effective sample preparation is essential for reliable long-chain fatty acid analysis, encompassing lipid extraction, fractionation, and derivatization. The following techniques address critical preprocessing requirements:
Lipid Extraction Methodologies
| Method | Advantages | Limitations | Optimal Applications |
|---|---|---|---|
| Folch | High recovery (>95%) | Chloroform/methanol toxicity | Small-scale research |
| (CHCl₃:MeOH 2:1) | Broad lipid compatibility | Environmentally impactful | Basic lipidomics |
| MTBE | Simplified phase separation | Slightly reduced recovery | High-throughput screening |
| 96-well plate compatibility | Large-scale studies | ||
| SPE | Chain-length selectivity (C18) | Stringent purity requirements | Isomer separation |
| Isomer resolution (Ag⁺ columns) | Suboptimal for polar FAs | Precision lipidomics |
Derivatization Strategies for Chromatographic Analysis
| Reagent | Target Group | GC | LC-MS | Key Characteristics |
|---|---|---|---|---|
| BF₃/MeOH | Carboxyl | ★★★★ | ✕ | Reference methyl esterification (FAME) |
| BSTFA | Hydroxyl/Carboxyl | ★★★ | ★★ | Dual-function silylation |
| NHPH | Carboxyl | ✕ | ★★★★ | 100× LC-MS sensitivity enhancement |
Critical Technical Notes
- BF₃/MeOH Derivatization:
- Applications: GC-optimized FAME generation
- Constraints: LC-MS incompatibility
- BSTFA Silylation:
- Advantages: Improved volatility (GC) & polarity (LC)
- Considerations: Complex protocol; potential bioactivity alteration
- NHPH Aminophosphorylation:
- Strengths: Ultra-sensitive LC-MS detection
- Constraints: GC incompatibility; specialized reagent requirements
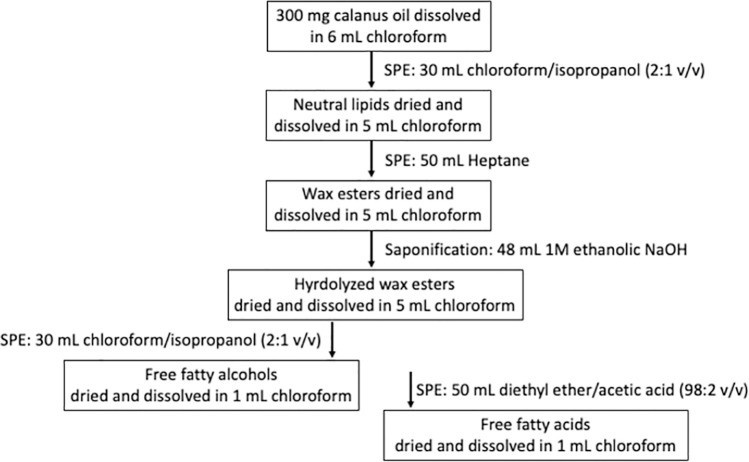 Isolation of fatty acids and fatty alcohols in wax esters present in Calanus oil by use of solid phase extraction (Schots PC et al., 2023)
Isolation of fatty acids and fatty alcohols in wax esters present in Calanus oil by use of solid phase extraction (Schots PC et al., 2023)
Precision Quantification Breakthroughs
1. Isomer Resolution Systems
(1) Geometric Isomer Separation
- GC Optimization Framework:
- Column Chemistry: CP-Sil 88 stationary phase (100% cyanopropyl polysiloxane) leverages:
- π-π interactions with double bonds
- Differential van der Waals forces (cis-trans retention discrimination)
- Temperature Gradient:
- 60°C (2min) → 1°C/min → 120°C → 3°C/min → 180°C → 5°C/min → 220°C (10min)
- Enhances C18:1 trans/cis resolution (Rₛ:1.2→1.8)
- Carrier Gas Effects:
- Flow (mL/min) Theoretical Plates Resolution
- Column Chemistry: CP-Sil 88 stationary phase (100% cyanopropyl polysiloxane) leverages:
| Flow (mL/min) | Theoretical Plates | Resolution |
|---|---|---|
| 0.8 | 85,000 | 1.5 |
| 1.0 | 72,000 | 1.2 |
| 1.2 | 60,000 | 0.9 |
(2) Positional Isomer Identification
- LC-MS/MS Advanced Workflow:
- Collision Energy Optimization:
- Prescan precursor selection
- CE gradient testing (10-35eV)
- Characteristic fragment selection (3 ions)
- DP/CE parameter refinement
- Diagnostic Fragment Library:
Fatty Acid Precursor (m/z) Fragments (m/z) Optimal CE C18:1n9 281.2 59.0/113.1 18 eV C20:4n6 303.2 259.1/205.0 25 eV C22:6n3 327.2 229.1/283.2 28 eV - 2D Separation: C18 RP-LC (chain length) → Chiralpak IA (n-3/6/9 isomers)
- Collision Energy Optimization:
2. Isotope Dilution Mass Spectrometry (IDMS)
Internal Standard Design:
- Labeling Principles:
- ¹³C distal to carboxyl (derivatization stability)
- D at ω-position (metabolic persistence)
- Tiered Standard System:
- ¹³C₁₆-C16:0 (extraction control)
- D₃-C18:0 (derivatization monitor)
- ¹³C₂₂-C22:6n3 (ionization verification)
Calibration & Validation:
| Parameter | Specification |
|---|---|
| Calibration Model | Weighted quadratic regression (1/x²) |
| QC Tiers | LLOQ: CV<20% (±25%) |
| Low: CV<15% (±20%) | |
| Med/High: CV<10% (±15%) | |
| Sensitivity | LLOQ: 0.1 pg/μL (plasma) |
| Stability | RT: <5% Δ/24h; -80°C: <10% Δ/3mo |
3. Emerging Quantification Platforms
| Technology | Implementation | Performance Gains |
|---|---|---|
| MRM³ (QTRAP) | Q1→CE1→Q2→CE2→Q3 fragment detection | 10× specificity (C18:2n6c) |
| Orbitrap PRM | 140K res (@m/z400); <1ppm mass error | Sub-fmol sensitivity |
| Microfluidic Chip-MS | Online derivatization→enrichment→nanoESI | <1μL sample; <5min/analysis |
Application Scenario Guide
Cardiovascular Risk Prediction
Very long-chain saturated fatty acids (VLSFA) demonstrate significant clinical utility for cardiovascular disease prevention and management. Epidemiological evidence reveals an inverse correlation between plasma phospholipid VLSFA concentrations and cardiovascular risk: individuals in the highest quartile of 24:0 levels exhibit 33% reduced heart failure incidence (95% CI: 19-45%) and 32% lower atrial fibrillation risk (15-45%).
For analytical quantification, GC-MS with a DB-23 capillary column is recommended. The optimized temperature program initiates at 60°C (2 min hold) followed by ramping to 220°C. Method validation requires:
- Retention time variation for 24:0 < 0.1 minutes
- Internal standard recovery maintained at 85-115%
Clinically, we propose a risk stratification model where plasma 24:0 < 1.2% combined with a 22:0/16:0 ratio < 0.05 indicates elevated cardiovascular risk (AUC = 0.78). Dietary supplementation of 200-300 mg/day VLSFA is advised, achievable through macadamia oil (1.8% 20:0 content) or deep-sea fish roe (2.3% 22:0 content) (Lemaitre RN et al., 2022).
Preterm birth risk assessment
Risk Association Strength:
A significant inverse relationship exists between EPA+DHA concentrations and preterm delivery risk:
- Quintile 1 (<1.6%): 10.27-fold risk elevation (95%CI:6.80-15.79)
- Quintile 2 (1.6-1.8%): 2.86-fold increased risk (1.79-4.59)
Threshold analysis revealed steep risk escalation below 2.0% EPA+DHA, stabilizing above 2.5%. - Temporal Dynamics:
- Strong concordance (r=0.82) between early (9-week) and mid-pregnancy (25-week) measurements. Persistently low concentrations conferred maximal risk (OR=48.0, p<0.001).
Methodology:
- GC-FID analysis employed:
- SP-2560 capillary column (100m × 0.25mm)
- Temperature gradient: 90°C → 250°C
- Hydrogen carrier gas flow: 1.3 mL/min
- Quality Assurance:
- Intra-batch precision: EPA CV=1.8%, DHA CV=1.9%
- Calibration with NIST-certified reference materials
- Sample Handling Protocol:
- Collection: 4 mL EDTA-anticoagulated whole blood
- Processing: Plasma isolation at 4°C, storage at -80°C
- Stability: <5% degradation after 3-year cryopreservation
Clinical Risk Stratification
- Intervention Framework:
| PA+DHA Concentration | Risk Category | Clinical Management |
|---|---|---|
| <1.6% | Extremely High | Immediate supplementation + weekly monitoring |
| 1.6-2.0% | High | Nutritional intervention + monthly follow-up |
| >2.5% | Low | Routine prenatal surveillance |
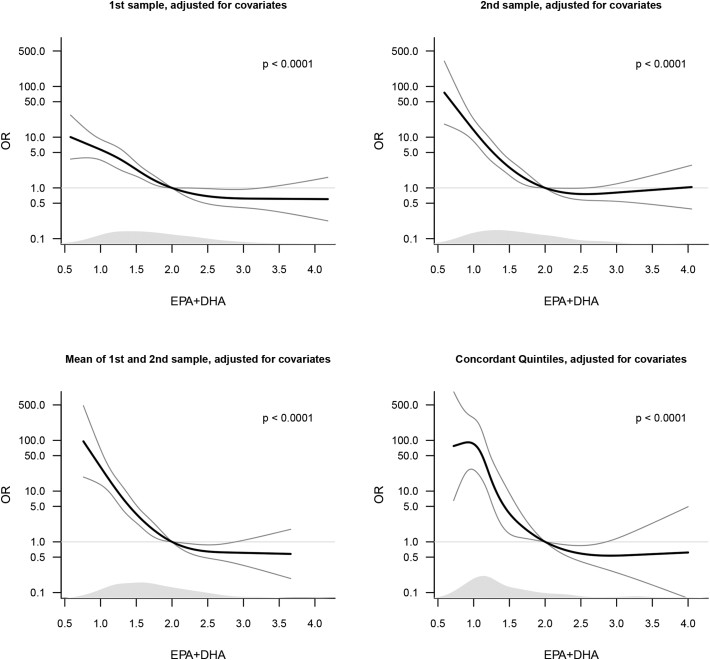 EPA+DHA measured values (Olsen SF et al., 2020)
EPA+DHA measured values (Olsen SF et al., 2020)
Fish Oil Quality Evaluation
Analytical Methodology
- Employed dual-platform analysis:
- GC-FID with fatty acid methylation
- HPLC-UV with anisidine value determination
- Key specifications:
- SP-2560 capillary column (100m × 0.25mm)
- Temperature gradient: 90°C → 250°C (5°C/min ramp)
- Inter-laboratory CV <5% (AOCS-certified)
Principal Research Outcomes
- Oxidation Profile Analysis
- Seasoning Interference: 63% of 25 seasonings induced p-AV false positives. Resolution: Developed tandem-anisidine value (TAV) HPLC-UV detection.
- Storage Impact:
Duration PV Increase DHA Retention 12 months +35% 98.2% 24 months +78% 95.6% - Label Accuracy Verification
- Comparative compliance rates:
- Current multi-laboratory study: 91%
- Albert et al. (single lab): 9%
- Comparative compliance rates:
Clinical Relevance
- Quality-Efficacy Correlation
- Bioavailability:
- Qualified products (PV<5): 23% higher EPA absorption (p<0.01)
- Oxidized products (TOTOX>26): Inhibit blood-brain barrier DHA transport
- Bioavailability:
- Dose-Response Relationship
- Products with ≥90% labeled EPA+DHA content yield:
- 15% cardiovascular risk reduction
- 12% cognitive improvement
- Products with ≥90% labeled EPA+DHA content yield:
- Clinical Stratification Guidance
| Oxidation Level | Target Population | Dosage Recommendation |
|---|---|---|
| TOTOX <20 | Pregnant women/children | Standard dose |
| 20-26 | Healthy adults | 20% dose elevation |
| >26 | Not recommended | Contraindicated |
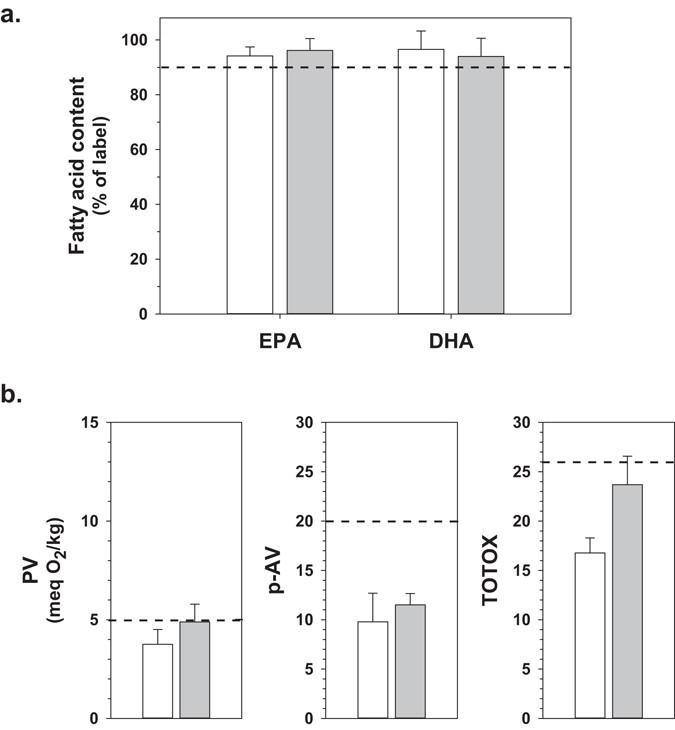 Content of EPA/DHA and oxidative status of several New Zealand fish oil (Bannenberg G et al., 2017)
Content of EPA/DHA and oxidative status of several New Zealand fish oil (Bannenberg G et al., 2017)
To learn more about the role of long-chain fatty acids in human health, please refer to "The Role of Long-Chain Fatty Acids in Human Health and Disease".
To learn how fatty acid metabolism can accelerate drug development, please refer to "How Fatty Acid Metabolomics Can Accelerate Your Drug Discovery Pipeline".
Standardization and Quality Assurance
1. Certified Reference Materials
- NIST SRM 1950: Provides certified concentrations for 42 plasma LCFAs
- ERM-BB186: Definitive fish oil fatty acid composition standard
2. Interlaboratory Validation Metrics
| Parameter | Acceptance Criteria |
|---|---|
| Extraction Recovery | 85-115% |
| Intra-day Precision | RSD <10% |
| Linear Range | ≥3 orders of magnitude |
3. Comprehensive QC Protocol
- Process Workflow:
- Sample collection with antioxidants (BHT/EDTA)
- -80°C storage (≤3 months)
- Extraction recovery verification
- Internal standard calibration (R²>0.995)
- Daily system suitability testing
4. International Method Benchmarking
| Standard | Matrix | Advantages | Limitations |
|---|---|---|---|
| AOAC 996.06 | Food | High-throughput (96-well) | Isomer resolution deficiency |
| ISO 12966-2 | Oils (plant/animal) | Geometric isomer separation | Requires 100m capillary |
| CLSI C62-A | Clinical specimens | Trace detection (LOQ=0.1ng/mL) | Instrumentation costs |
5. Data Standardization Framework
- Normalization Methods:
- Isotope dilution (¹³C-FA internal standard)
- Total LCFA normalization (∑[C14-C22]=100%)
- Database Integration:
- Automated LipidMAPS identifier matching
- HMDB metabolic pathway annotation
Cutting-Edge Technological Advances
1. Single-Cell Fatty Acid Omics
Integrated Workflow Optimization:
- Cell Capture:
- Microfluidic 10μm channels (>95% efficiency)
- Optical integrity monitoring (CV<5%)
- Nano-Extraction Methods:
Technique Recovery Key Advantage Nanopore filtration 92% Compatible with <1μL volumes Electromagnetic-assisted 88% Charge-based FA enrichment - High-Resolution MS:
- Orbitrap Elite parameters:
Resolution: 240,000 @ m/z 400
Scan range: m/z 150-1000
AGC Target: 1e6
- Orbitrap Elite parameters:
Key Findings:
- Hepatocellular carcinoma subpopulations exhibit:
- 14-fold C16:0 variation (25-350 amol/cell)
- Metastatic correlation: ω-3/ω-6 ratio (0.12-1.8)
2. Spatial Imaging Breakthroughs
MALDI-TOF IMS Enhancements:
- Novel Matrices:
- DHB: Optimal for C12-C20 FAs
- Norharmane: 3-5× signal boost for >C20
- Multimodal Integration:
- Atherosclerosis Insights:
- Fibrous cap C20:4n6 gradient: core>periphery (p<0.001)
- Spatial MMP-9 expression correlation (r=0.82)
3. AI-Driven Analytical Expansion
- DeepLCFA v2.0 Architecture:
- Input: MS1/MS2 + RT + CE → Hidden: 3-layer LSTM (512 nodes) + Attention → Output: LipidMAPS ID + Confidence
- Performance Metrics:
Parameter v1.0 v2.0 Identification 92.7% 96.3% False Positive 7.5% 3.2% Throughput 10/hr 50/hr - Innovative Applications:
- Real-time MS calibration (Δm/z<0.5ppm)
- Metabolic enzyme activity prediction (ACC/FASN)
4. Convergent Technology Platforms
Microfluidics-MS Integration:
- Chip Architecture:
- Cell culture → Stimulation → Extraction → Nano-ESI
| Specification | Performance |
|---|---|
| Dead volume | <50 nL |
| Parallel channels | 96 |
| Detection limit | 10 zmol |
Cryo-EM Innovations:
- Workflow: Rapid vitrification (<1ms) → Cryo-FIB-SEM → In situ MALDI
- Lipid Droplet Findings:
- C18:1n9 crystalline phase (<100nm resolution)
- Phase-specific FA quantification
References
- Chiu HH, Kuo CH. "Gas chromatography-mass spectrometry-based analytical strategies for fatty acid analysis in biological samples." J Food Drug Anal. 2020 Jan;28(1):60-73. doi: 10.1016/j.jfda.2019.10.003
- Weatherly CA, Zhang Y, Smuts JP, Fan H, Xu C, Schug KA, Lang JC, Armstrong DW. "Analysis of Long-Chain Unsaturated Fatty Acids by Ionic Liquid Gas Chromatography." J Agric Food Chem. 2016 Feb 17;64(6):1422-32. doi: 10.1021/acs.jafc.5b05988
- De Biase I, Pasquali M. "Quantification of Very-Long-Chain and Branched-Chain Fatty Acids in Plasma by Liquid Chromatography-Tandem Mass Spectrometry." Methods Mol Biol. 2022;2546:509-521. doi: 10.1007/978-1-0716-2565-1_46
- Vargas-Muñoz MA, Cerdà V, Turnes Palomino G, Palacio E. "Determination of long-chain fatty acids in anaerobic digester supernatant and olive mill wastewater exploiting an in-syringe dispersive liquid-liquid microextraction and derivatization-free GC-MS method." Anal Bioanal Chem. 2021 Jun;413(15):3833-3845. doi: 10.1007/s00216-021-03338-z
- Lemaitre RN, King IB. "Very long-chain saturated fatty acids and diabetes and cardiovascular disease." Curr Opin Lipidol. 2022 Feb 1;33(1):76-82. doi: 10.1097/MOL.0000000000000806
- Schots PC, Edvinsen GK, Olsen RL. "A simple method to isolate fatty acids and fatty alcohols from wax esters in a wax-ester rich marine oil." PLoS One. 2023 May 12;18(5):e0285751. doi: 10.1371/journal.pone.0285751
- Olsen SF, Halldorsson TI, Thorne-Lyman AL, Strøm M, Gørtz S, Granstrøm C, Nielsen PH, Wohlfahrt J, Lykke JA, Langhoff-Roos J, Cohen AS, Furtado JD, Giovannucci EL, Zhou W. "Plasma Concentrations of Long Chain N-3 Fatty Acids in Early and Mid-Pregnancy and Risk of Early Preterm Birth. EBioMedicine. 2018 Sep;35:325-333. doi: 10.1016/j.ebiom.2018.07.009. Epub 2018 Aug 3." Erratum in: EBioMedicine. 2020 Jan;51:102619. doi: 10.1016/j.ebiom.2018.07.009
- Bannenberg G, Mallon C, Edwards H, Yeadon D, Yan K, Johnson H, Ismail A. "Omega-3 Long-Chain Polyunsaturated Fatty Acid Content and Oxidation State of Fish Oil Supplements in New Zealand." Sci Rep. 2017 May 3;7(1):1488. doi: 10.1038/s41598-017-01470-4









