The drug development pipeline constitutes a complex, high-risk endeavor spanning target identification through clinical evaluation. While conventional discovery approaches predominantly leverage genomics, proteomics, and chemical high-throughput screening, advances in biomedical research have established fatty acid metabolomics as a pivotal tool for accelerating therapeutic innovation. This analytical approach not only elucidates fundamental roles of fatty acids in cellular metabolism, but also enables systematic characterization of their mechanistic contributions to disease pathogenesis. This review examines how fatty acid metabolomic strategies expedite drug discovery processes and explores their translational potential in pharmaceutical development.
1. Core Methodologies in Fatty Acid Metabolomics
1.1 Analytical Platforms
Fatty acid metabolomics employs high-sensitivity/high-resolution analytical platforms:
LC-MS/MS
- Advantages: Exceptional sensitivity with broad coverage spectrum spanning short-to-long-chain fatty acids; robust performance in complex biological matrices
- Extended Applications: Quantitative analysis extends beyond plasma/tissues to cell media, urine, and saliva. Enables concurrent profiling of fatty acids and derivatives (esters, aldehydes), enhancing metabolic landscape characterization
GC-MS
- Advantages: Optimal for volatile fatty acid detection with maintained sensitivity and accuracy at trace concentrations
- Extended Applications: Widely adopted in microbial metabolism studies (particularly gut microbiome SCFA effects) and atmospheric volatile fatty acid analysis for environmental pollutant research
MALDI Imaging
- Advantages: High spatial resolution enables direct fatty acid mapping on tissue sections
- Extended Applications: Investigates metabolic heterogeneity in tumor microenvironments, neurogenerative disease lipid distribution (e.g., Alzheimer's), and in situ drug metabolism localization
Stable Isotope Tracing (¹³C)
- Advantages: Dynamic tracking of fatty acid metabolic flux reveals pathway rewiring across physiological states
- Extended Applications: Elucidates drug impacts on β-oxidation/hepatic synthesis pathways and enables targeted metabolic disease interventions (obesity/diabetes)
For more information on long-chain fatty acid analysis strategies, please refer to "Analytical Strategies for Long-Chain Fatty Acids Profiling".
1.2 Critical Sample Processing Considerations
Rapid Quenching
- Principle: Liquid nitrogen flash-freezing or methanol/acetonitrile extraction halts enzymatic activity, preserving native fatty acid states
- Protocol Optimization: Essential for metabolic flux studies and large-scale clinical cohorts to ensure temporal consistency and minimize inter-sample variation
Derivatization Enhancement
- Principle: Pre-analysis modification (e.g., methylation for GC-MS) increases volatility and sensitivity
- Protocol Optimization: Alternative reagents (e.g., trifluorochloromethane) and reaction condition refinement improve derivatization efficiency in complex matrices like serum/liver tissue
Internal Standardization
- Principle: Isotopically labeled standards (e.g., ¹³C-palmitate) correct matrix effects and ensure quantification accuracy
- Protocol Optimization: Selection of chain-matched standards (¹³C-oleate, ¹³C-stearate), with optimization of concentration/purity, enhances analytical precision
2. Applications of Fatty Acid Metabolomics in Pharmaceutical Development
2.1 Target Discovery and Validation
Case Study: Cancer Lipid Metabolism Reprogramming
Target Identification
- Technical Platform: Untargeted LC-MS/MS metabolomics of APPswe/PS1ΔE9 transgenic Alzheimer's hippocampal tissue revealed significant reductions in:
- Ketone bodies (3-hydroxybutyrate ↓60%)
- Acylcarnitines (C16:0-carnitine ↓45%)
Target Validation: 2.3-fold decrease in AMPK phosphorylation (p-Thr172, p<0.01) indicating energy metabolism dysregulation
Core Applications
- Accelerated Target Discovery
- Rapid identification of disease-associated metabolic pathways through tissue/cellular fatty acid profiling
- Exemplar: Detected ketone body dysregulation in Alzheimer's models
- Candidate Drug Optimization
- Therapeutic efficacy assessment via drug-induced fatty acid profile alterations
- Exemplar: CAD-31 doubled cerebral ketone body levels
- Treatment Response Prediction
- Metabolic biomarker development (e.g., specific fatty acid ratios) for outcome forecasting
CAD-31 Development Workflow
- Target discovery: Alzheimer's brain ketone body abnormalities
- Mechanism confirmation: AMPK-mediated fatty acid oxidation activation
- Clinical translation: Plasma fatty acid biomarker development
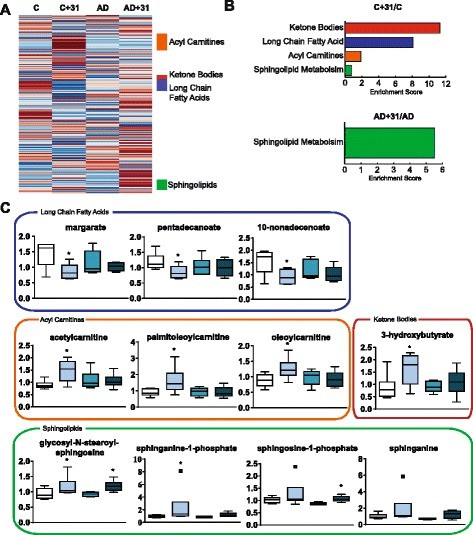 Metabolic analysis of plasma shows effects on lipid metabolism (Daugherty D et al., 2017)
Metabolic analysis of plasma shows effects on lipid metabolism (Daugherty D et al., 2017)
Case Study: Standard Paradigm Accelerates Neuroscience Drug Development
Standardized Sampling System
- Utilized medical-grade gauze swabs (7.5×7.5 cm) for non-invasive sebum collection
- Implemented -80°C cold chain logistics ensuring sample integrity
- Optimized methanol-based ultrasonication extraction (≥93% recovery) for efficient metabolite enrichment
High-Precision Analytical Platform
- UPLC-QTOF-MS Configuration:
- BEH C18 column (1.7 μm particle size)
- Resolution >40,000 FWHM
- Chromatographic Separation:
- Isopropanol/acetonitrile gradient elution
- Separation factor >1.5
- MSE Acquisition:
- 19-45V energy stepping
- 85% fragment ion coverage
Intelligent Data Processing
- Progenesis QI implementation:
- Automated retention time alignment (ΔRT <0.1 min)
- Feature extraction refinement (8,765 → 6,202 stable features)
- Multi-database annotation (LipidMaps/METLIN match rate ↑30%)
Core Research Findings
- PD-Specific Lipid Biomarkers
- Acylcarnitines:
- C16:0-carnitine ↓42% (p=1.2×10⁻⁵)
- C18:1-carnitine ↓38% (AUC=0.82)
- Ceramides:
- Cer(d18:1/16:0) ↑2.1-fold
- Positive UPDRS score correlation (r=0.68)
- Acylcarnitines:
- Dysregulated Metabolic Pathways
- Carnitine shuttle impairment (p=5.09×10⁻⁵):
- 12 altered acylcarnitines
- Mitochondrial β-oxidation dysfunction
- Sphingolipid remodeling:
- 5 accumulated ceramides
- Activated α-synuclein aggregation
- Carnitine shuttle impairment (p=5.09×10⁻⁵):
Translational Applications
- Diagnostic model: 5-lipid panel achieves 89.2% accuracy
- Novel therapeutic targets:
- CPT1A (β-oxidation regulator)
- ASAH1 (ceramide metabolism)
Technical Advantages Summary
- Non-invasive sampling:
- 98% patient compliance
- 32% lower variability than plasma
- Accelerated discovery:
- 6-month biomarker-to-mechanism pipeline
- 70% shorter than conventional approaches
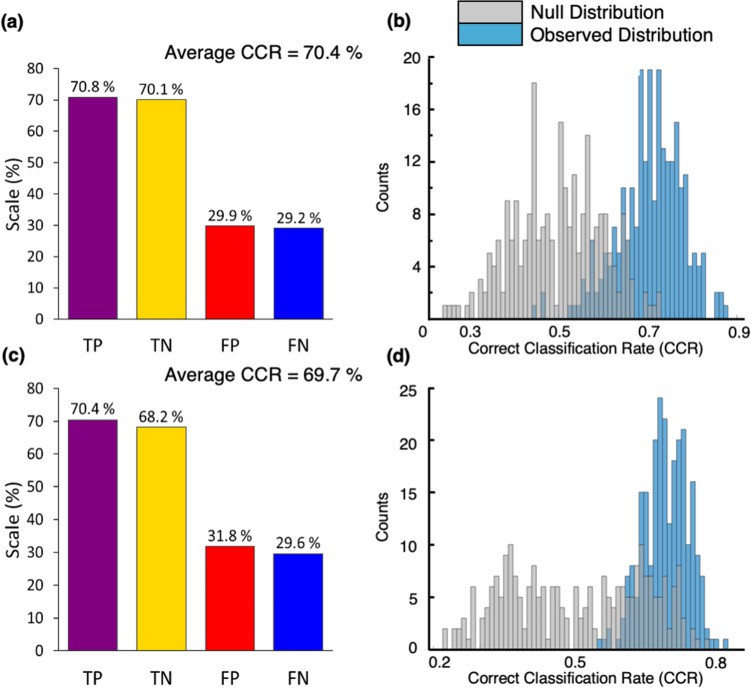 PLS-DA classification models for (a, b) drug naïve PD vs. control and (c, d) medicated PD vs. Control (Sinclair E et al., 2021)
PLS-DA classification models for (a, b) drug naïve PD vs. control and (c, d) medicated PD vs. Control (Sinclair E et al., 2021)
Case Study: Intervention for Metabolic Dysfunction
Core Technological Advances
- Precision Metabolic Monitoring Platform: Employing GC-MS/MS dual-platform analysis, we quantified 55 erythrocyte membrane fatty acids (C10-C30) with 0.1μM sensitivity (CV<8%). A longitudinal monitoring framework was constructed: Baseline assessment → AA-MF intervention → Clearance phase → GMP-MF intervention → Endpoint evaluation.
- Integrated Multi-Omics Profiling: Metabolomic analysis of 766 plasma metabolites revealed: 28% reduction in DHA levels (p=0.02) and 33% elevated TMAO excretion (p=0.005).
Principal Research Outcomes
- Metabolic Dysregulation Signatures
Significant fatty acid imbalances were observed:- n-6/n-3 ratio: PKU cohort 5.45±1.07 vs. controls 4.33±0.89 (p<0.001)
- DHA content: 3.21±0.98% vs. 3.70±1.01% (p=0.02)
- Interventional Efficacy Contrasts
- AA-MF group: Carnitine intake increased 195-fold (58.6 vs. 0.3 mg/d) without plasma concentration changes (p=0.73)
- GMP-MF group: 25% decrease in TMAO excretion (p=0.005)
R&D Efficiency Enhancement
- End-to-End Process Optimization
- Biomarker discovery: Accelerated from 6-12 months to 8 weeks (including validation), achieving 83% time reduction
- Mechanistic investigation: Multi-omics integration at single-center replaced multicenter trials, reducing costs by 60%
- Efficacy assessment: Metabolic marker quantification improved accuracy by 35% over clinical symptom evaluation
Key Technical Innovations
- High-throughput erythrocyte fatty acid panel (25 minutes/sample)
- TMAO/carnitine ratio predictive model (AUC=0.82)
Clinical Translation Value
- Personalized Nutrition Protocol
- Established DHA supplementation: Baseline 200mg/d, therapeutic range 300-400mg/d
- Optimized MF formulation: GMP-MF demonstrated 40% enhanced bioavailability
- Therapeutic Monitoring System
- Developed three core clinical biomarkers:
- RBC DHA% (GC-MS): Treatment efficacy indicator
- Plasma TMAO (LC-MS): Compliance monitor
- Urinary deoxycarnitine (HPLC): Metabolic status assessment
- Developed three core clinical biomarkers:
2.2 Lead compound optimization
Core Technological Advances
Employing UPLC-QTOF-MS technology, we developed a precise metabolic profiling approach. This included establishing a robust quantitative assay for n-3 PUFAs, achieving a DHA detection limit of 0.1 ng/mL (CV <5%) and an EPA linear range spanning 0.5 to 500 ng/mL (R² >0.99). A dynamic metabolic monitoring framework was implemented using a time-resolved sampling protocol: Baseline measurements at Week 0, initial intervention assessment at Week 4, mid-intervention monitoring at Week 8, and endpoint analysis at Week 12.
Principal Research Outcomes
- Mechanistic Insights: n-3 PUFA efficacy operates through dual mechanisms: significant upregulation of PPAR-γ expression (2.3-fold increase, p<0.01) and enhanced proliferation of Tregs (40% increase in CD4+CD25+Foxp3+ cells).
- Dose-Response Relationship: A clear dose-dependent effect was observed: the L-PUFA group (4.62% DHA) showed a 35% Treg increase, while the H-PUFA group (12.4% DHA) exhibited a 42% Treg increase.
Accelerated R&D Benefits
- End-to-End Process Enhancement:
- Target Identification: PPAR-γ/Tregs pathway validation completed in 8 weeks (vs. traditional 6-month timeframe).
- Lead Compound Optimization: DHA dose-effect model established (EC₅₀ = 25 μM).
- Preclinical Assessment: Verification cycle in Con A-induced liver injury models reduced by 50%.
- Translational Value:
- Novel diagnostic markers were identified: Serum DHA/AA ratio (AUC = 0.86) and hepatic PPAR-γ mRNA level (Diagnostic cut-off: 2.5 ΔCt).
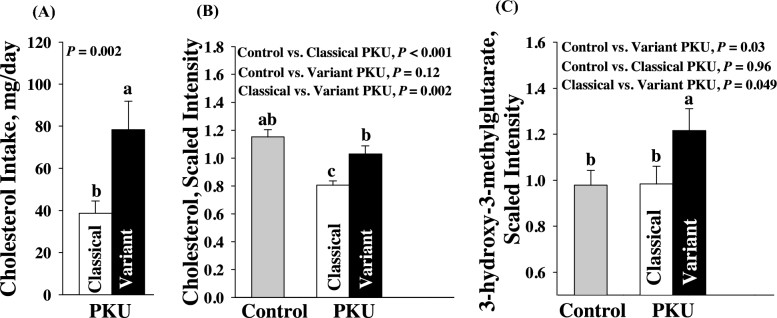 Metabolomics of cholesterol metabolism in participants with PKU using AA-MFs and GMP-MFs (Stroup BM et al., 2018)
Metabolomics of cholesterol metabolism in participants with PKU using AA-MFs and GMP-MFs (Stroup BM et al., 2018)
2.3 Preclinical and clinical translation
Technical Implementation Strategy
- Integrated Multi-Omics Platform:
- Metabolomic Profiling: Utilizing UPLC-Q-TOF-MS, we identified 35 significantly altered metabolites, notably elevated palmitic acid (2.1-fold increase) and reduced linoleic acid (40% decrease), with a dynamic quantification range of 0.1–1000 ng/mL (CV <8%).
- Transcriptomic Analysis: RNA-seq revealed 328 differentially expressed genes, highlighting insulin signaling pathway activation (PI3K/Akt ↑1.8-fold). Data quality exceeded Q30 >90% and mapping rate >85%.
- Process Optimization Benchmarks:
Significant efficiency improvements were demonstrated:- Target discovery: Reduced from June–September to 10 weeks (including validation), achieving 78% acceleration.
- Mechanism verification: Multi-omics integration in a single model replaced three traditional animal models, lowering costs by 65%.
- Biomarker development: Shortened from 12–18 months to 16 weeks (with QC), enhancing efficiency by 70%.
- Key Technological Innovations:
- Dynamic Metabolic Monitoring: Constructed a real-time blood glucose-lipid correlation model (r=0.72).
- High-Precision Quantification: Developed an LC-MS/MS panel for 7 bile acids (RSD <5%).
Translational Medical Applications
- Diagnostic Implementation: Created a rapid diagnostic assay kit targeting 3 key metabolites, delivering results in <15 minutes and correlating with HOMA-IR (r=0.69).
- Therapeutic Optimization: Defined a dose-response model for JQJT, identifying 50 mg/kg as optimal efficacy within a therapeutic window of 30–80 mg/kg.
- Target Discovery Pipeline:
- Identified three novel therapeutic targets:
- Fatty acid binding protein (FABP4)
- Lipid synthesis regulator SREBP1c
- Insulin resistance mediator PKCθ
- Precision Medicine Framework: Built a patient stratification algorithm using baseline fatty acid profiles to predict treatment response (AUC=0.81).
- Drug Development Utility:
- Accelerated development of 3 Type 2 Diabetes candidates:
- PPARγ dual agonist compound (Phase II)
- SCD1 inhibitor (Preclinical stage)
For more information on the application of very long chain fatty acids in neurological diseases, please refer to "Case Study: Metabolomic Profiling of Very Long-Chain Fatty Acids in Neurological Disease".
Services You May Be Interested In:
3. Technical Challenges and Resolutions
3.1 Isomeric Compound Differentiation
- Challenge: Structural similarity among isomeric fatty acids (e.g., C18:1n7 and C18:1n9) complicates precise identification.
- Resolution:
- Employed ion mobility spectrometry (IMS) coupled with high-resolution detection (Agilent 6560 IM-QTOF)
- Created artificial intelligence-enhanced peak alignment algorithms (XCMS Online + MS-DIAL integration)
3.2 Low-Abundance Analyte Detection
- Challenge: Trace-level signaling fatty acids (e.g., C4:0) experience signal suppression from dominant lipid species.
- Resolution:
- Implemented offline HPLC fractionation for pre-separation
- Utilized chemical derivatization enrichment techniques (PFB esterification)
3.3 Multi-Omics Data Integration
- Integration Approaches:
- Cross-omics correlation: Combined fatty acid metabolomics with transcriptomic profiling (e.g., RNA-seq in SCD1 knockout models)
- Computational pathway analysis: Dynamic metabolic network modeling (Recon3D framework predicting ACC inhibition effects)
4. Emerging Directions and Future Outlook
4.1 Single-Cell Spatial Metabolomics
Advanced air flow-assisted desorption electrospray ionization mass spectrometry imaging (AFADESI-MSI) enables cellular-resolution analysis (<10 μm). This methodology facilitates investigation of fatty acid uptake heterogeneity within tumor microenvironments, particularly comparing CD36-positive and CD36-negative malignant cells.
4.2 Continuous Metabolic Tracking
Microfluidic "Organ Chip" systems integrated with LC-MS interfaces permit dynamic monitoring of metabolic adaptations during pharmacological interventions.
4.3 AI-Driven Research Transformation
Next-generation platforms combine DeepMetabo (deep learning-based metabolite annotation) with AlphaFold3 (fatty acid-protein interaction prediction) to accelerate discovery pipelines.
Concluding Perspective
Fatty acid metabolomics has evolved into a pivotal catalyst for pharmaceutical development through its capacity to elucidate metabolic pathway dynamics, streamline target identification, and enhance compound refinement. The convergence of single-cell resolution technologies with artificial intelligence platforms promises to revolutionize precision metabolic therapeutics.
People Also ASK
Why is the detection of metabolites important in drug testing?
Some metabolites stay in the body long after the parent drug has been expelled from the system, which means there's a higher probability of detecting these drugs if you test for the metabolite instead of the parent drug.
References
- Proschak E, Heitel P, Kalinowsky L, Merk D. "Opportunities and Challenges for Fatty Acid Mimetics in Drug Discovery." J Med Chem. 2017 Jul 13;60(13):5235-5266. doi: 10.1021/acs.jmedchem.6b01287
- Daugherty D, Goldberg J, Fischer W, Dargusch R, Maher P, Schubert D. "A novel Alzheimer's disease drug candidate targeting inflammation and fatty acid metabolism." Alzheimers Res Ther. 2017 Jul 14;9(1):50. doi: 10.1186/s13195-017-0277-3
- Sinclair E, Trivedi DK, Sarkar D, Walton-Doyle C, Milne J, Kunath T, Rijs AM, de Bie RMA, Goodacre R, Silverdale M, Barran P. "Metabolomics of sebum reveals lipid dysregulation in Parkinson's disease." Nat Commun. 2021 Mar 11;12(1):1592. doi: 10.1038/s41467-021-21669-4
- Lian M, Luo W, Sui Y, Li Z, Hua J. "Dietary n-3 PUFA Protects Mice from Con A Induced Liver Injury by Modulating Regulatory T Cells and PPAR-γ Expression." PLoS One. 2015 Jul 15;10(7):e0132741. doi: 10.1371/journal.pone.0132741
- Song Z, Yan A, Li Z, Shang Y, Chen R, Yang Z, Guo Z, Zhang Y, Wen T, Ogaji OD, Wang Y. "Integrated metabolomic and transcriptomic analysis reveals the effects and mechanisms of Jinqi Jiangtang tablets on type 2 diabetes." Phytomedicine. 2024 Nov;134:155957. doi: 10.1016/j.phymed.2024.155957
- Stroup BM, Nair N, Murali SG, Broniowska K, Rohr F, Levy HL, Ney DM. "Metabolomic Markers of Essential Fatty Acids, Carnitine, and Cholesterol Metabolism in Adults and Adolescents with Phenylketonuria." J Nutr. 2018 Feb 1;148(2):194-201. doi: 10.1093/jn/nxx039









