1. Fundamental Concepts and Structural Attributes of Long-Chain Fatty Acids
2. Hierarchical Classification of Long-Chain Fatty Acids
3. Structure-Function Interrelationships in Fatty Acids
4. Physiological Networks of Long-Chain Fatty Acid Functions
5. Structure-Function
Summary
Long-chain fatty acids (LCFAs), defined as aliphatic carboxylic acids containing 12-22 carbon atoms, represent the predominant category of fatty acids in human physiology. These compounds fulfill critical roles across nutritional, biochemical, and metabolic domains, serving as the primary constituents of dietary lipids.
1. Fundamental Concepts and Structural Attributes of Long-Chain Fatty Acids
1.1 Definition and Classification Criteria
Long-chain fatty acids (LCFAs) are defined as aliphatic carboxylic acids comprising 12-22 carbon atoms, representing the predominant fatty acid category in biological systems. Classification follows International Union of Biochemistry (IUPAC) standards:
- Short-chain: C2-C5 (e.g., acetic, propionic acids)
- Medium-chain: C6-C11 (e.g., caprylic, capric acids)
- Long-chain: C12-C22 (e.g., lauric to docosahexaenoic acids)
- Very-long-chain: ≥C23 (e.g., nervonic acid)
1.2 Core Structural Organization
While sharing a common carboxyl-terminal framework, LCFAs exhibit distinctive features through chain elongation:
- Polar headgroup: Carboxyl moiety (-COOH)
- Ionizes to -COO⁻ at physiological pH
- Confers amphiphilic properties
- Forms ester bonds in glyceride synthesis
- Hydrocarbon chain:
- Composed of methylene (-CH₂-) units
- Chain length governs hydrophobicity
- C12-C22 range enables substantial hydrophobic interactions
- ω-Terminus (methyl end):
- Serves as carbon numbering origin
- Basis for ω-3/6/9 classification
- Site for specific oxidative modifications
1.3 Dimensions of Structural Diversity
LCFA heterogeneity manifests through four critical parameters:
- Chain length: C12-C22 carbon permutations
- Degree of unsaturation: Saturated to polyunsaturated states
- Double bond position: Determines metabolic fate and functionality
- Stereoconfiguration: cis vs. trans isomeric forms
1.4 Topology of carbon chain determines physical properties
- Saturated linear chains (e.g., stearic acid, C18:0) display a high melting point (69°C) and marked hydrophobicity. Van der Waals interactions produce tightly packed crystalline arrangements, creating the rigid structural foundation essential for biofilms.
- In contrast, unsaturated chains with bends (e.g., docosahexaenoic acid/DHA, C22:6) contain ≥3 cis double bonds that induce 30–60° kinks. This configuration diminishes molecular packing order, substantially increasing membrane fluidity by approximately 300%.
- Meanwhile, extra-long saturated chains (e.g., lignoceric acid, C24:0) exhibit heightened hydrophobic character due to chain elongation. This promotes specialized "lipid raft" domain formation within sphingolipids, modulating membrane protein functionality.
1.5 The polar head group serves dual roles:
- As a hydrogen bond donor, the carboxyl group (-COOH) facilitates targeted binding to receptor proteins like GPR120.
- Double bond positioning critically influences biological activity: Omega-3 fatty acids (e.g., eicosapentaenoic acid/EPA) feature their first unsaturation at the third carbon from the methyl terminus, enabling anti-inflammatory effects; conversely, omega-6 compounds (e.g., arachidonic acid) possess their initial double bond at the sixth position, acting as pro-inflammatory precursors.
For more on the difference between long-chain fatty acids and short-chain fatty acid, see "Long-Chain vs. Medium- and Short-Chain Fatty Acids: What's the Difference?".
For more information on long-chain fatty acids, please refer to "What Are Long-Chain Fatty Acids? A Beginner's Guide".
2. Hierarchical Classification of Long-Chain Fatty Acids
2.1 Saturation-Based Categorization
2.1.1 Saturated Fatty Acids (SFAs)
Structural hallmark: Absence of carbon-carbon double bonds
Key representatives:
- Lauric acid (12:0): Primary constituent in coconut oil
- Myristic acid (14:0): Abundant in dairy lipids
- Palmitic acid (16:0): Predominant natural SFA
- Stearic acid (18:0): Major component of animal fats
Physical behavior:
- Melting point elevation with chain elongation
- Typically solid at ambient temperature
- Significant van der Waals interactions
2.1.2 Monounsaturated Fatty Acids (MUFAs)
Structural signature: Single double bond
Notable examples:
- Palmitoleic acid (16:1n-7): Fish oils and select plants
- Oleic acid (18:1n-9): >75% composition in olive oil
- Erucic acid (22:1n-9): Historically high in rapeseed cultivars
Bond attributes:
- Predominantly cis configuration
- Endogenous synthesis via desaturase enzymes
2.1.3 Polyunsaturated Fatty Acids (PUFAs)
Structural definition: ≥2 double bonds
Subclass representatives:
- Dienoic: Linoleic (18:2n-6)
- Trienoic: α-Linolenic (18:3n-3)
- Tetraenoic: Arachidonic (20:4n-6)
- Hexaenoic: DHA (22:6n-3)
Biological roles:
- Essential fatty acid carriers
- Eicosanoid biosynthesis precursors
- Critical membrane phospholipid constituents
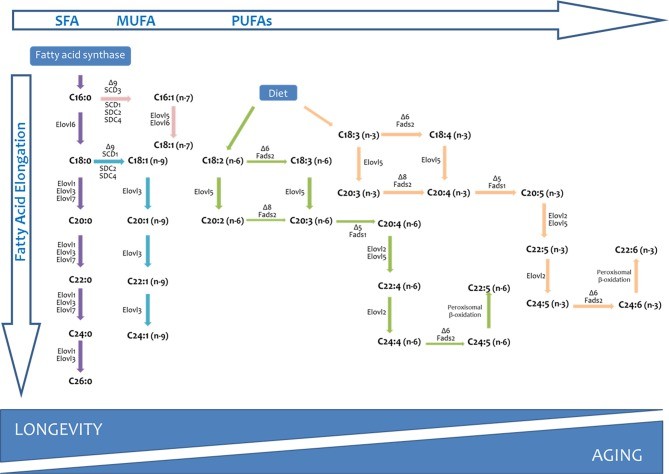 Long chain and very long-chain fatty acid biosynthesis in vertebrates (Naudí A et al., 2013)
Long chain and very long-chain fatty acid biosynthesis in vertebrates (Naudí A et al., 2013)
2.2 Double Bond Position Classification
2.2.1 ω-3 Series
Metabolic precursor: α-Linolenic acid (ALA, 18:3n-3)
Elongation metabolites:
- EPA (20:5n-3): Potent anti-inflammatory agent
- DPA (22:5n-3): Neural protection mediator
- DHA (22:6n-3): Brain/retinal structural component
Metabolic regulation:
- Δ6-desaturase as rate-limiting enzyme
- Competitive inhibition with ω-6 pathway
2.2.2 ω-6 Series
Primary substrate: Linoleic acid (LA, 18:2n-6)
Biosynthetic derivatives:
- γ-Linolenic (GLA, 18:3n-6)
- Dihomo-γ-linolenic (DGLA, 20:3n-6)
- Arachidonic (AA, 20:4n-6)
Functional attributes:
- Pro-inflammatory eicosanoid precursors
- Homeostatic balance with ω-3 compounds
2.2.3 ω-7/ω-9 Series
Characteristic members:
- Palmitoleic (16:1n-7)
- Oleic (18:1n-9)
- Mead (20:3n-9)
Metabolic features:
- Endogenously synthesized
- Upregulated during essential FA deficiency
2.3 Geometric Isomer Differentiation
2.3.1 cis-Unsaturated FAs
Natural configuration:
- Syn-orientation of hydrogen atoms
- ~30° chain bending at double bond
- Reduced molecular packing efficiency
Nutritional relevance:
- Cardioprotective lipids in olive oil/fish oil
- Demonstrated cardiovascular benefits
2.3.2 trans-Unsaturated FAs
Formation pathways:
- Industrial partial hydrogenation
- Rumen microbial biohydrogenation
Structural consequences:
- Anti-orientation of hydrogen atoms
- Linear chain conformation retention
- SFA-like physicochemical behavior
Health impacts:
- Elevate LDL-cholesterol concentrations
- Suppress HDL-cholesterol levels
- Promote inflammatory responses
3. Structure-Function Interrelationships in Fatty Acids
3.1 Carbon Chain Length Determinants
3.1.1 Absorption and Transport Mechanisms
MCFAs:
- Portal vein direct absorption
- Bile-independent uptake
- Rapid energy substrate utilization
LCFAs:
- Micellar solubilization prerequisite
- Intracellular re-esterification to triglycerides
- Chylomicron-mediated transport
- Lymphatic systemic entry
3.1.2 Metabolic Pathway Specificity
β-Oxidation efficiency:
- C6-C10: Mitochondrial direct oxidation
- C12-C20: Carnitine shuttle-dependent
- ≥C22: Peroxisomal primary oxidation
Tissue distribution patterns:
- C16-C18: Ubiquitous tissue incorporation
- ≥C20: Selective tissue partitioning (e.g., neural accretion)
3.2 Double Bond Configuration Effects
3.2.1 Membrane Biophysical Modulation
Fluidity regulation:
- Saturated FAs elevate membrane order
- Each unsaturation decreases phase transition by 5-10°C
- DHA reduces bilayer thickness ≈15%
Microdomain organization:
- Saturated FAs stabilize lipid rafts
- ω-3 PUFAs disrupt raft integrity
- Signal transduction modulation
3.2.2 Oxidative Vulnerability
Structural susceptibility:
- Per-oxidation rate increases exponentially with unsaturation count
- Conjugated dienes exhibit enhanced reactivity
- ω-3 > ω-6 oxidation propensity
Stabilization requirements:
- Vitamin E co-supplementation for PUFA-rich diets
- Aldehydic toxin generation during thermal processing
3.3 Stereochemical Functional Implications
3.3.1 cis-Configuration Advantages
Biological functionality:
- Optimal membrane fluidity maintenance
- Membrane protein conformational support
- Lipid mediator bioavailability
Metabolic features:
- Lipase recognition efficiency
- High oxidative metabolic yield
- Reduced vascular deposition
3.3.2 trans-Fat Pathogenicity
Molecular deception mechanisms:
- Saturated FA-like metabolism
- Retained unsaturation reactivity
Disease pathogenesis:
- Essential FA metabolic interference
- Atherogenic plaque potentiation
- Insulin sensitivity impairment
4. Physiological Networks of Long-Chain Fatty Acid Functions
4.1 Energy Metabolism Regulation
4.1.1 Optimized Energy Storage
Caloric density comparison (per gram):
- Lipids: 9 kcal
- Proteins/carbohydrates: 4 kcal
- Ethanol: 7 kcal
Storage efficiency:
- Anhydrous deposition
- 1 kg adipose tissue ≈ 7,700 kcal
- Sustains adult basal metabolism for 72-96 hours
4.1.2 Metabolic Homeostasis
Lipolytic control:
- Hormone-sensitive lipase modulated by insulin/glucagon
- Stress-induced activation
- Liberates circulating free fatty acids
Oxidative regulation:
- Carnitine palmitoyltransferase-mediated transport
- AMPK pathway modulation
- Reciprocal inhibition with glucose metabolism
4.2 Cellular Membrane Architecture
4.2.1 Phospholipid Asymmetry
sn-1 position:
- Saturated FA dominance (e.g., palmitic/stearic acids)
- Membrane structural stabilization
sn-2 position:
- Unsaturated FA enrichment (e.g., arachidonic/DHA)
- Fluid-phase maintenance
4.2.2 Membrane-Mediated Signaling
Receptor modulation:
- GPCR conformational dynamics
- Ion channel gating probability
- Receptor dimerization states
Signal transduction:
- Lipid raft compartmentalization
- Secondary messenger precursor generation
- Kinase spatial organization
4.3 Bioactive Lipid Precursors
4.3.1 Eicosanoid Biosynthesis
Production cascade:
- Phospholipase A2-mediated AA/EPA release
- Cyclooxygenase → Prostaglandins
- Lipoxygenase → Leukotrienes
Functional antagonism:
- ω-6 derivatives: Pro-inflammatory mediators
- ω-3 derivatives: Anti-inflammatory resolvers
- Homeostatic inflammation control
4.3.2 Endocannabinoid System
Precursor conversion:
- AA → Anandamide (AEA)/2-AG
- DHA → Docosahexaenoyl ethanolamide (DHEA)
Neuromodulatory effects:
- ω-6 metabolites: Anxiogenic properties
- ω-3 metabolites: Neuroprotective functions
- Cognitive-emotional regulation
4.4 Genomic Regulation Mechanisms
4.4.1 Nuclear Receptor Activation
PPAR isoforms:
- PPARα: FA catabolism induction
- PPARγ: Adipogenesis promotion
- PPARδ: Metabolic equilibrium
LXR/RXR heterodimers:
- Cholesterol homeostasis
- Bile acid biosynthesis
- Glucose metabolism involvement
4.4.2 Epigenetic Modulation
Histone modifications:
- Acetyl-CoA substrate provision
- HDAC inhibition by SCFAs
Non-coding RNA regulation:
- ω-3 suppression of oncogenic miR-21
- Tumor suppression pathway modulation
- Fibrotic progression control
Services You May Be Interested In:
5. Structure-Function
Regulation of virulence: Pathogens like Salmonella and Clostridium difficile directly modulate virulence factor secretion (e.g., toxins, adhesins). Enteric pathogens encounter a dynamic nutritional landscape shaped by host secretions, microbial metabolism, and dietary components. Critically, metabolites—collectively termed long-chain fatty acids (LCFAs)—exhibit biogeographical concentration gradients along intestinal segments (e.g., jejunum versus colon), creating an environmental signaling network detected by pathogens. LCFAs suppress virulence via dual pathways:
- Allosteric Inhibition: Direct binding to virulence gene transcriptional activators (e.g., FadR proteins) diminishes their DNA affinity, thereby repressing virulence expression.
- Signaling Cascade Disruption: By altering histidine kinase receptor activity (e.g., EnvZ/OmpR system), LCFAs modify downstream phosphorylation cascades to inhibit virulence programs. Exemplifying this:
- Palmitic acid (C16:0) reduces Salmonella intestinal invasion by 40-60% via Hi1A/Hi1D two-component system inhibition
- Oleic acid (C18:1) downregulates the ToxT virulence regulator in Vibrio cholerae, suppressing toxin production (Mitchell MK et al., 2022).
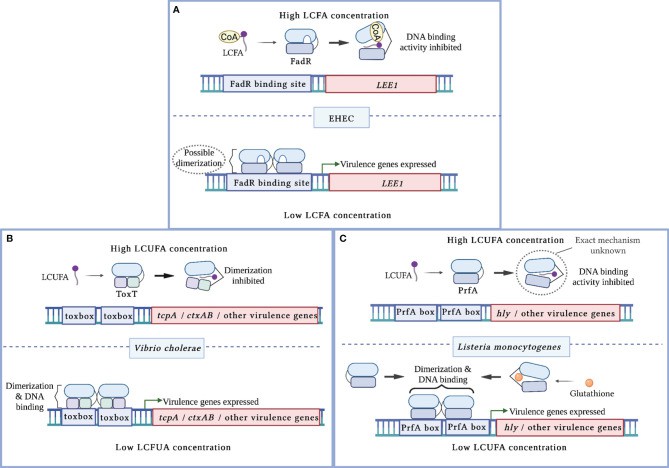 Mechanisms of virulence regulation by LCFA cytoplasmic sensors (Mitchell MK et al., 2022)
Mechanisms of virulence regulation by LCFA cytoplasmic sensors (Mitchell MK et al., 2022)
Long-chain fatty acids (LCFAs) exhibit bidirectional immunomodulatory effects in inflammatory bowel disease, critically regulating immune-inflammatory equilibrium through two primary mechanisms:
1. Signaling Pathway Regulation
- Anti-inflammatory actions: Suppression of proinflammatory pathways via TLR4/MyD88/NF-κB axis downregulation, reducing TNF-α and IL-6 release; NLR inflammasome inhibition, preventing IL-1β maturation.
- Pro-resolution actions: PPAR-γ pathway upregulation enhances anti-inflammatory mediators (e.g., IL-10); Elevated resolvin and protectin biosynthesis.
2. Immune Cell Functional Reprogramming
Macrophage repolarization toward M2 anti-inflammatory phenotypes; T cell lineage modulation: Th17 suppression and Treg functional enhancement; Neutrophil recruitment attenuation through reduced CXCL1/CXCL8 chemokine expression.
3. Dynamic Intestinal Barrier Modulation
- Protective mechanisms: Tight junction reinforcement via claudin-1/occludin upregulation; Goblet cell stimulation with consequent MUC2-mediated mucus hypersecretion; Epithelial regeneration potentiation through Wnt/β-catenin activation.
- Pathological risks: Saturated LCFAs (e.g., palmitic acid) can: Trigger endoplasmic reticulum stress; Elevate epithelial cell apoptotic rates; Compromise mucus layer structural integrity (Ma C etal., 2019).
VLCFAs leverage their pronounced hydrophobicity and structural heterogeneity to execute critical plant functions across four domains:
- Physical Barrier Formation
Cuticular and cork-associated waxes comprise intricate blends of VLCFA derivatives (aldehydes, alkanes, ketones) integrated with non-acyl cyclic compounds including terpenoids and flavonoids. - Membrane Functional Modulation
VLCFAs fine-tune signal perception and protein anchoring. Wattelet-Boyer et al. [161] demonstrated via transmission electron microscopy that sphingolipid-VLCFA chain length governs trans-Golgi network (TGN) secretory vesicle morphology and connectivity in Arabidopsis roots. Critically, sphingolipids enable de novo polar sorting of auxin transporter PIN2 to apical membranes. This establishes a molecular linkage whereby TGN-localized VLCFA derivatives facilitate gravitropic responses through PIN2 trafficking in polarized cells. - Developmental and Energetic Programming
Anther endothelial expression of KCS6 and CER2 homologs is essential for generating ≥C26 VLCFAs. These lipid derivatives accumulate on pollen surfaces, functioning as hydration signals that activate σ-cell-mediated water transport during germination. - Environmental Adaptation Evolution
VLCFAs underpin disease, drought, and heavy metal resistance. Arabidopsis KCS1 mutants exhibit impaired seedling survival under low humidity [56], whereas *BnKCS1-1* or *BnKCS1-2* overexpression enhances cuticular wax deposition in rapeseed (Brassica napus), reducing transpirational water loss and improving drought tolerance (Batsale M et al., 2021).
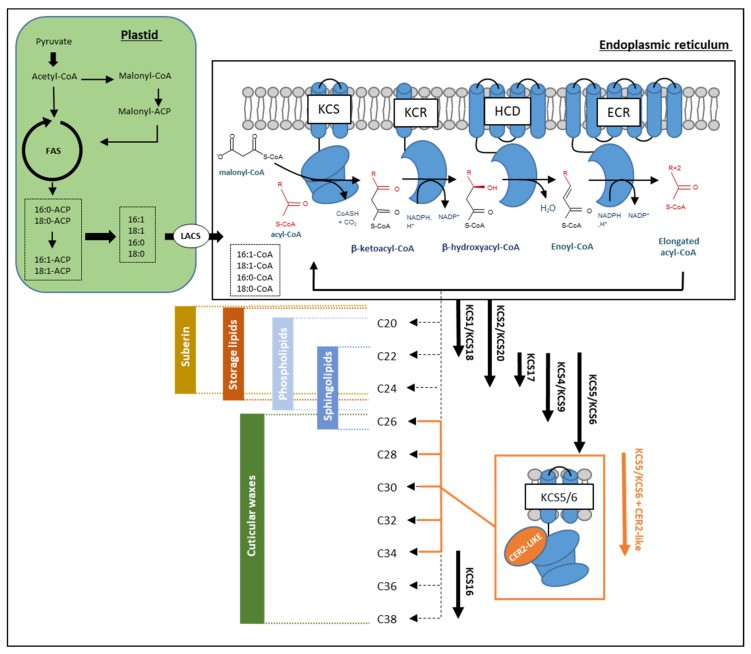 Biosynthesis and selective involvement of VLC acyl-CoAs in the different lipid biosynthesis pathways in Arabidopsis (Batsale M et al., 2021)
Biosynthesis and selective involvement of VLC acyl-CoAs in the different lipid biosynthesis pathways in Arabidopsis (Batsale M et al., 2021)
Summary
| Structural Feature | Physicochemical Effect | Core Biological Function | Application Example |
| Long-Chain Saturated Structure | High melting point, strong hydrophobicity | Membrane stability, energy storage | Palm oil structural lipids |
| Polyunsaturated Bending | Low melting point, high fluidity | Membrane dynamics, anti-inflammatory signaling | Fish oil for cardiovascular disease prevention |
| Very Long-Chain (>C22) | Extreme hydrophobicity, high rigidity | Cellular barrier, developmental signaling | Stress-resistant crop breeding |
| Carboxyl Polar Head | Amphiphilicity, self-assembly into membranes | Basic structure of lipid bilayers | Artificial membrane simulation systems |
The biological significance of long-chain fatty acids is rooted in their precise carbon chain configurations. Future research will further elucidate the dynamic interactions between structure and function, providing targets for metabolic disease treatment, functional lipid design, and synthetic biology.
For more on the role of long-chain fatty acids in human disease, read "The Role of Long-Chain Fatty Acids in Human Health and Disease".
References
- Naudí A, Jové M, Ayala V, Portero-Otín M, Barja G, Pamplona R. "Membrane lipid unsaturation as physiological adaptation to animal longevity." Front Physiol. 2013 Dec 17;4:372. doi: 10.3389/fphys.2013.00372
- Agbaga MP, Ahmad M. "Emerging insights into the function of very long chain fatty acids at cerebellar synapses." Neural Regen Res. 2025 Jun 1;20(6):1709-1710. doi: 10.4103/NRR.NRR-D-24-00436
- Lauritzen L, Brambilla P, Mazzocchi A, Harsløf LB, Ciappolino V, Agostoni C. "DHA Effects in Brain Development and Function." Nutrients. 2016 Jan 4;8(1):6. doi: 10.3390/nu8010006
- Mitchell MK, Ellermann M. "Long Chain Fatty Acids and Virulence Repression in Intestinal Bacterial Pathogens." Front Cell Infect Microbiol. 2022 Jun 17;12:928503. doi: 10.3389/fcimb.2022.928503
- Ma C, Vasu R, Zhang H. "The Role of Long-Chain Fatty Acids in Inflammatory Bowel Disease." Mediators Inflamm. 2019 Nov 3;2019:8495913. doi: 10.1155/2019/8495913
- Batsale M, Bahammou D, Fouillen L, Mongrand S, Joubès J, Domergue F. "Biosynthesis and Functions of Very-Long-Chain Fatty Acids in the Responses of Plants to Abiotic and Biotic Stresses." Cells. 2021 May 21;10(6):1284. doi: 10.3390/cells10061284


