Glutathione Protein Modification Service
To support researchers investigating oxidative signaling and protein function, we offer a comprehensive Protein Glutathione Sites Identification Service. Using advanced LC-MS/MS techniques combined with enrichment strategies tailored to detect glutathionylated peptides, we help uncover specific glutathione modification sites with high sensitivity and precision.
- Site-Specific Identification: Confident localization of glutathionylation sites on target proteins.
- High Sensitivity & Specificity: Enrichment protocols enhance detection even at low modification levels.
- Data Interpretation Support: Bioinformatics tools provide insights into affected pathways and protein networks.
- GSH and GSSG Quantification: Accurate measurement of reduced and oxidized glutathione levels to assess cellular redox status.
This service is ideal for Redox Proteomics research, stress response studies, and PTM characterization in plant and mammalian systems alike.
Submit Your Request Now
×
- Precise identification of protein glutathionylation sites.
- Quantitative comparison and functional enrichment analysis.
- Publication-ready graphs and full data reports.
- Optional data interpretation and bioinformatics support.
- What is
- Detection Methods
- Service Details
- FAQ
- Case Study
- Publication
- Demo
What is Glutathionylation
Glutathione, a tripeptide composed of γ-glutamyl, cysteinyl, and glycine units, plays a central role in cellular redox homeostasis. It exists in two forms: the reduced GSH and the oxidized GSSG, where two GSH molecules are linked by a disulfide bond. Predominantly found as GSH in living cells, this molecule participates in a range of critical biological functions, particularly in plant systems — including flowering, cellular differentiation, programmed cell death, immunity, symbiotic interactions, and cell cycle control.
One of the most important roles of glutathione is its involvement in a post-translational modification (PTM) process called glutathionylation. This modification, a subtype of S-thiolation, involves the formation of mixed disulfide bonds between glutathione and the cysteine residues of target proteins. While typically associated with oxidative stress conditions, glutathionylation also occurs under basal physiological conditions, especially during the regulation or regeneration of thiol-containing enzymes like peroxidases.
As a reversible PTM, glutathionylation is emerging as a key redox-based signaling mechanism that helps cells respond to environmental stress by regulating specific signaling cascades. It has been linked to the modulation of various stress-related pathways and is believed to play a pivotal role in cellular adaptation.
Primary Methods for Detecting Glutathionylated Proteins
Accurately detecting protein glutathionylation is essential for understanding redox-regulated cellular processes. Several analytical approaches have been developed, each with distinct advantages depending on the experimental context. Below are two widely used methods:
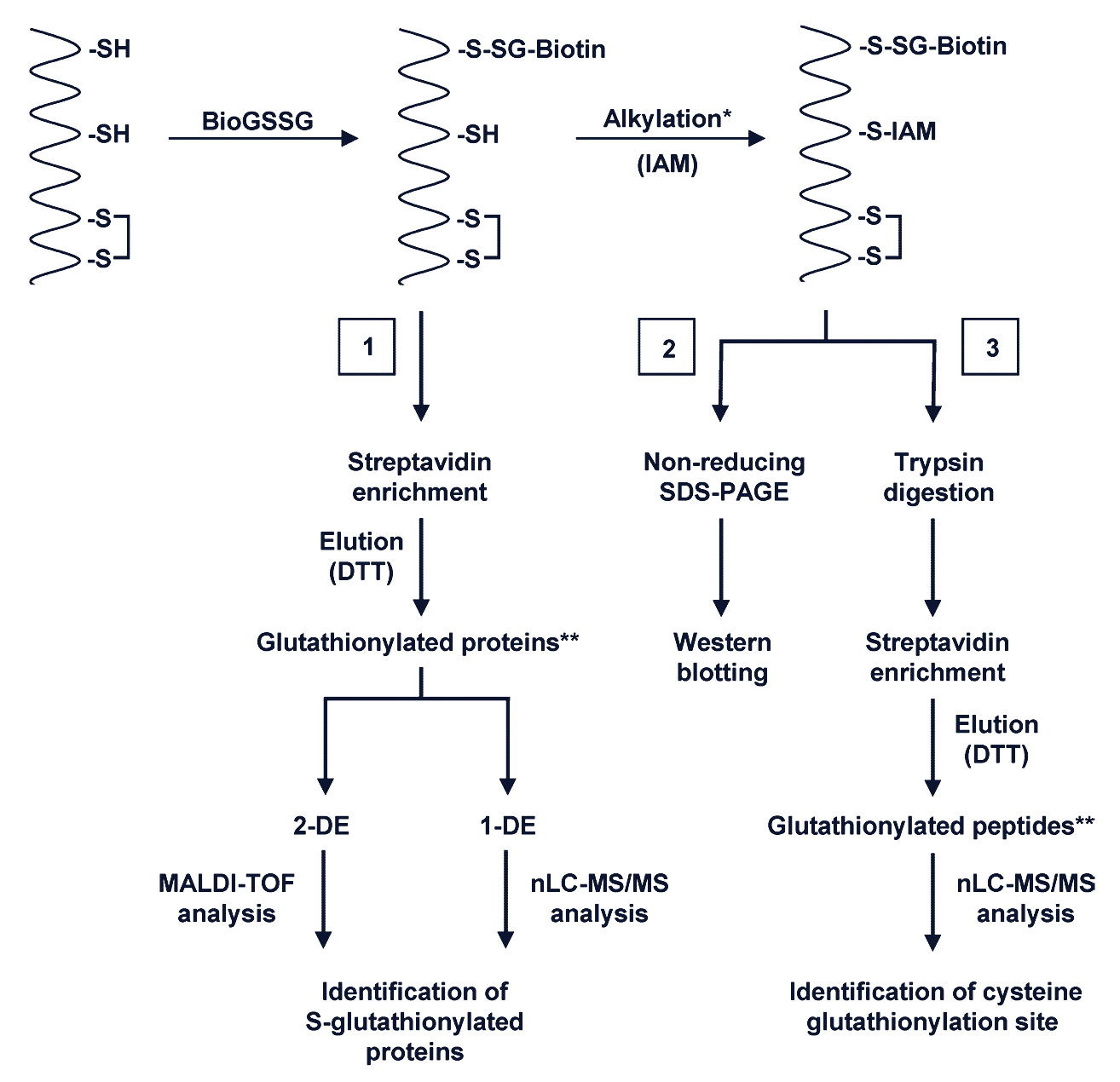
1. Biotinylated Glutathione Labeling
Biotin-conjugated glutathione reagents—such as BioGSH (reduced form), BioGSSG (oxidized form), and BioGEE (a cell-permeable ethyl ester analog)—can be synthesized in vitro and applied in both in vitro and in vivo settings. These probes allow for sensitive detection of glutathionylated proteins using:
- Non-reducing Western blotting
- Biotin-specific antibodies
- In vivo glutathionylation proteomics
Because of the biotin tag, labeled proteins can be easily enriched and visualized, making this technique particularly useful for proteome-scale studies.
 Anti-Glutathione Antibodies
Anti-Glutathione Antibodies
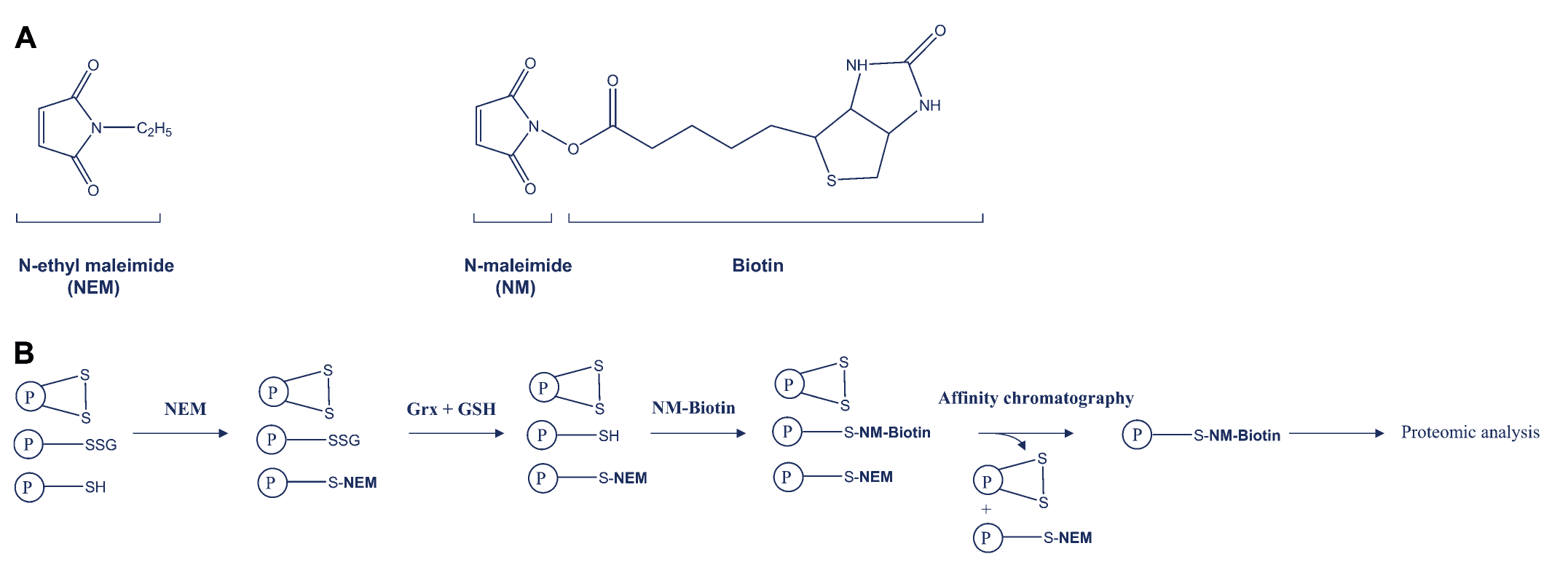
2. GRX-Based Selective Reduction and Enrichment
This chemical approach involves the following steps:
- Free thiols are first blocked using N-ethylmaleimide (NEM).
- Glutaredoxin (GRX) is then used to selectively reduce glutathionylated cysteines.
- Newly exposed thiols are labeled with NEM-biotin.
- The labeled proteins are purified via affinity chromatography and identified using mass spectrometry.
This method offers high specificity and is compatible with downstream proteomic workflows, enabling site-specific identification of glutathionylation.
 Workflow of GRX reduction of glutathionylated proteins
Workflow of GRX reduction of glutathionylated proteins
Each method offers distinct strengths—whether for targeted validation or comprehensive redox proteome mapping—and can be selected based on the study's scale, sensitivity needs, and sample type.
Our Protein Glutathione Sites Identification Service
At Creative Proteomics, we provide a streamlined and highly specialised service for identifying glutathione modification sites across a wide variety of biological samples—from mammalian tissues to microbial cultures. Our end-to-end workflow covers every step of the analysis process, enabling you to focus on interpreting the biological significance of your results.
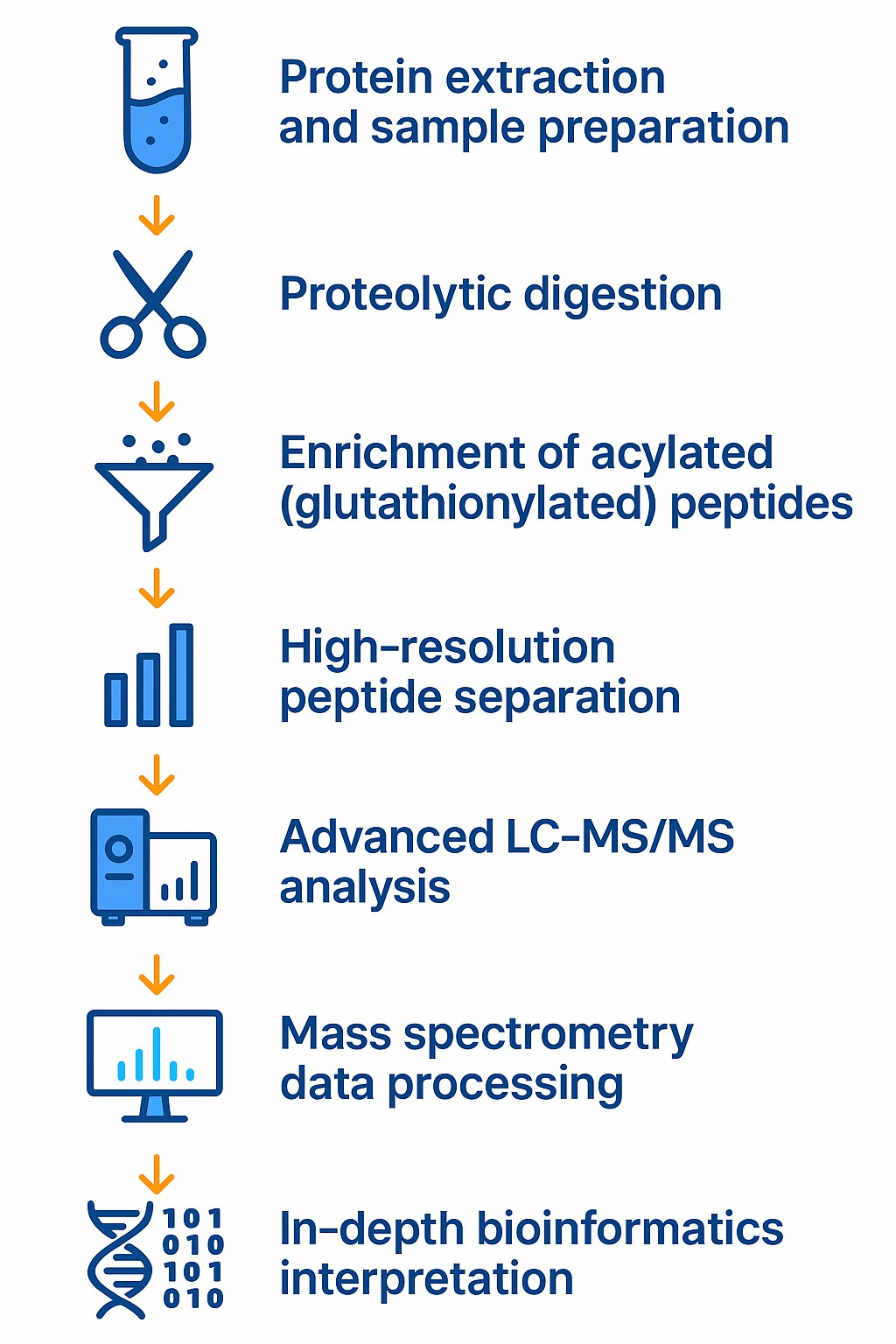
Our Comprehensive Workflow Includes:
- Protein extraction and sample preparation
- Proteolytic digestion
- Enrichment of acylated (glutathionylated) peptides
- High-resolution peptide separation
- Advanced LC-MS/MS analysis
- Mass spectrometry data processing
- In-depth bioinformatics interpretation
Whether you're investigating oxidative stress responses or mapping redox-regulated pathways, our platform delivers reliable, high-throughput results with exceptional precision.
Sample Requirements
We accept a broad range of sample types. Below are our minimum input guidelines:
| Sample Type | Minimum Quantity Required | Notes |
|---|---|---|
| Fresh animal tissue | ≥600 mg | – |
| Fresh plant tissue | ≥6 g | – |
| Cell cultures | ≥1 × 10⁷ cells/tube × 3 tubes | – |
| Fungi or bacteria | ≥600 mg | – |
| Serum or plasma | 450 μL × 4 tubes | – |
| Protein solution | 5–10 mg total protein | – |
| Body fluids | ≥15 mL (e.g., saliva, amniotic fluid, culture supernatant) | For urine: 15 mL × 4 tubes, centrifuge at 1000 × g for 5 min; discard pellet |
Why Choose Us?
High Enrichment Efficiency: Optimized protocols ensure selective capture of glutathionylated peptides.
Scalable Analysis: Capable of profiling glutathionylation at proteome scale using high-resolution mass spectrometry.
Quantitative Insights: Optional integration of commercial quantification techniques allows for comparative analysis across sample groups.
Our platform is ideal for researchers seeking reproducible, large-scale glutathionylation data to support studies in redox biology, signal transduction, or stress adaptation.
Glycine supplementation can partially restore oxidative stress-associated glutathione deficiency in ageing cats
Frequently Asked Questions (FAQ)
What can this service reveal about protein glutathionylation in my samples?
Our service provides precise identification of glutathionylated cysteine residues at the site-specific level. In addition to detecting modification sites, we offer optional quantification to assess changes in modification levels under different experimental conditions. This enables insights into redox-sensitive signaling events and stress-responsive pathways in biological systems.
Can this service quantify dynamic changes in glutathionylation across conditions or treatments?
Yes. We offer both relative and absolute quantification strategies to track dynamic glutathionylation changes across sample groups. Using label-free quantification or isotope-based approaches (e.g., TMT, SILAC, or spiked internal standards), we can compare modification intensities and generate fold-change data. This allows researchers to assess how glutathionylation fluctuates in response to oxidative stimuli, metabolic shifts, or genetic perturbations.
How are glutathionylated peptides enriched and identified?
We employ a GRX-based selective reduction protocol, where glutathionylated cysteines are specifically reduced and tagged for enrichment. The resulting peptides are analyzed using high-resolution LC-MS/MS. This strategy ensures high specificity for glutathione-modified peptides while maintaining sensitivity across a broad dynamic range.
What types of downstream data analysis do you provide?
We offer a full bioinformatics package including:
- Site and protein-level annotation
- Quantitative comparison tables
- GO/KEGG enrichment of modified proteins
- Subcellular localization prediction
- Motif analysis surrounding modification sites
- Optional protein-protein interaction network mapping
All results are provided in user-friendly formats and ready for figure generation.
Can I integrate these results with transcriptomic or metabolomic data?
Absolutely. We provide clean, structured data tables compatible with multi-omics analysis platforms. Whether you're correlating redox PTMs with gene expression, metabolic shifts, or pathway activity, our outputs can be directly integrated into your systems biology workflow.
How do you ensure that redox-sensitive modifications like glutathionylation are preserved?
Our protocols include sample handling under mild, reducing conditions and immediate thiol blocking using alkylating reagents (e.g., NEM) to prevent artificial oxidation. Sample processing is designed to preserve native redox states and reflect in vivo modification profiles as accurately as possible.
Is this service suitable for both plant and animal systems?
Yes. Our workflow is compatible with a wide range of sample types including mammalian tissues, plant materials, microbial cultures, and body fluids. We adjust buffer systems and enrichment conditions based on matrix type to optimize recovery and specificity.
What deliverables will I receive?
You will receive:
- A list of glutathionylated proteins and modification sites
- Quantitative comparison data (if requested)
- Functional enrichment analysis reports
- Publication-quality graphs and charts
- Full raw data and processed result files
- Optional interpretation summary upon request
Learn about other Q&A.
Client Case Study: Glutathione Restoration in Aging Cats through Glycine Supplementation

https://doi.org/10.1017/S0007114524000370
- Project Objective
- Our Contribution
- Key Findings
- Conclusion
Waltham Petcare Science Institute, in collaboration with Royal Canin, conducted a study to investigate the impact of aging on oxidative stress and glutathione metabolism in cats. The researchers observed that senior cats exhibited significantly reduced levels of erythrocyte and whole blood glutathione (GSH), along with elevated oxidative stress markers compared to younger counterparts.
To address this age-related glutathione deficiency, the team launched a controlled glycine (GLY) supplementation trial in senior cats. The goal was to assess whether dietary glycine could restore intracellular glutathione levels and reduce oxidative stress. To achieve this, the researchers partnered with Creative Proteomics for targeted quantification of erythrocyte glutathione and its oxidized form (GSSG).
Targeted Glutathione Quantification
Creative Proteomics provided a comprehensive glutathione quantification service, supporting the project with end-to-end technical execution:
- Sample Preparation: Erythrocyte pellets were processed under nitrogen, snap-frozen, and delivered to Creative Proteomics on dry ice.
- Isotopic Labelling: Internal standards containing ¹³C-labeled GSH and GSSG were prepared in antioxidant buffer to ensure quantitation accuracy.
- UPLC–QQQ-MS Analysis: Samples were analyzed using Agilent 1290 UPLC coupled with Agilent 6495B triple quadrupole mass spectrometry, operated in MRM mode for maximum specificity and sensitivity.
- Chromatographic Separation: A reverse-phase C18 column with a gradient elution profile was used to separate GSH and GSSG from other metabolites.
- Data Processing: Glutathione concentrations were determined by linear regression from standard curves, using the analyte-to-internal-standard peak ratios.
Our robust methodology ensured the reliable and reproducible detection of subtle changes in erythrocyte glutathione levels—critical for evaluating intervention efficacy.
Data generated by Creative Proteomics played a central role in uncovering the following outcomes:
- After 4 weeks of dietary glycine supplementation (1.5% free GLY), the test group showed a significant increase in erythrocyte total glutathione and GSH levels compared to the control group (P ≤ 0.03).
- Oxidative stress markers—8-OHdG and PGF2α—were significantly reduced at Weeks 8 and 12, confirming that improved glutathione levels correlated with reduced oxidative damage.
- The trend toward increased glutathione at Week 12 suggests sustained benefits of continued glycine supplementation.
- The study concluded that dietary glycine offers a promising strategy to counteract age-associated redox imbalance in felines.
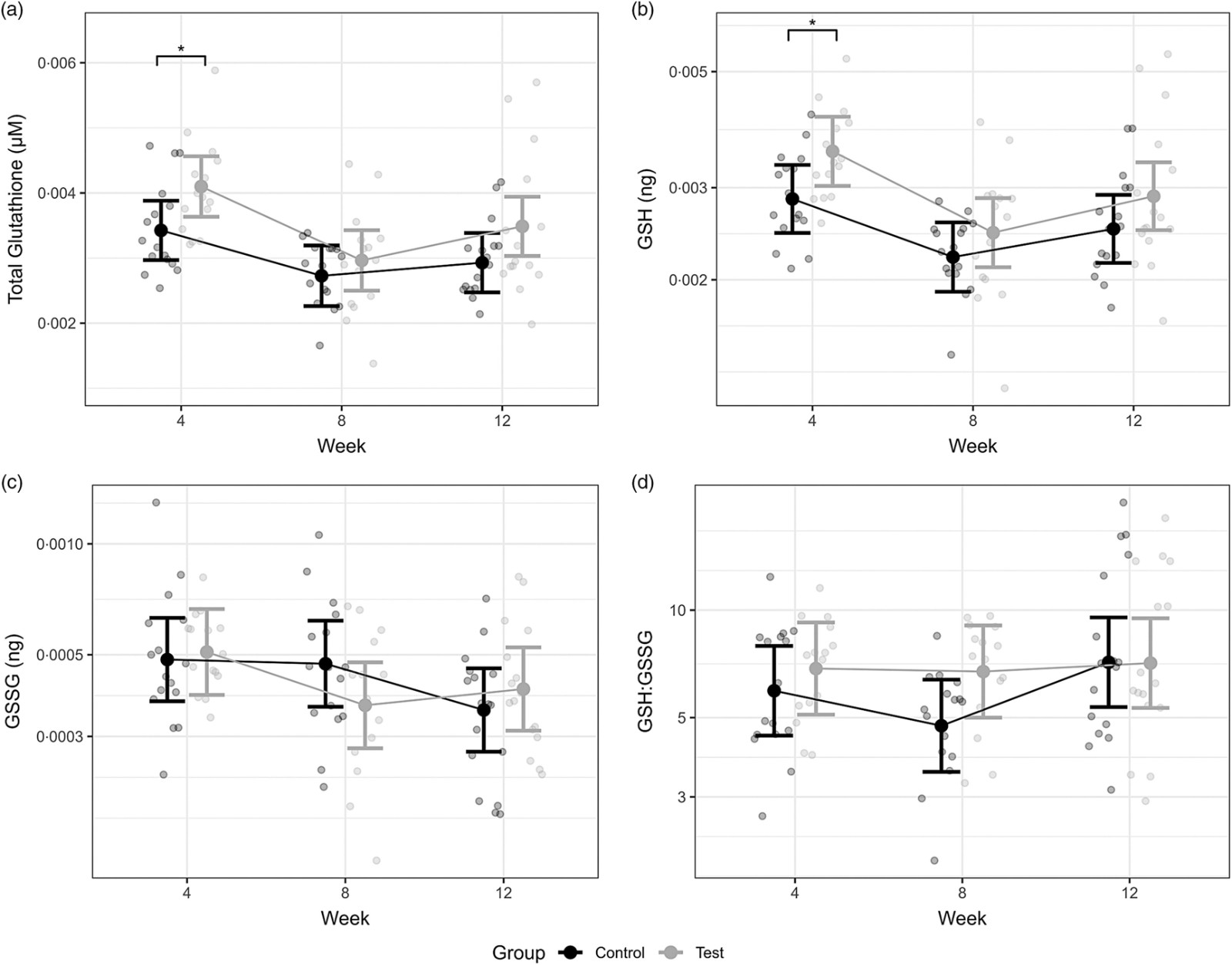 Figure 3. Creative Proteomics quantified significant increases in (a) total erythrocyte glutathione and (b) reduced glutathione (GSH) at Week 4 in cats receiving glycine-supplemented diets.
Figure 3. Creative Proteomics quantified significant increases in (a) total erythrocyte glutathione and (b) reduced glutathione (GSH) at Week 4 in cats receiving glycine-supplemented diets.
This case study highlights Creative Proteomics' technical excellence in glutathione and redox modification quantification. By providing isotope-labeled standards, precise LC-MS/MS workflows, and detailed data interpretation, we enabled our client to validate their hypothesis, publish their findings in the British Journal of Nutrition (2024), and propose dietary strategies for feline health.
Whether you're studying redox biology, metabolic aging, or amino acid interventions, Creative Proteomics is your trusted partner for high-resolution glutathionylation analysis.
Publications

- Glycine supplementation can partially restore oxidative stress-associated glutathione deficiency in ageing cats. British Journal of Nutrition. 2024. https://doi.org/10.1017/S0007114524000370
- Assessment of Fasciola hepatica glutathione S-transferase as an antigen for serodiagnosis of human chronic fascioliasis. Acta Tropica. 2018. https://doi.org/10.1016/j.actatropica.2018.07.002
Demo















