Trimetlylamine Oxide (TMAO) Analysis Service
Creative Proteomics offers a specialized TMAO quantification platform to unlock critical insights into gut microbiota activity, methylamine metabolism, and nutritional impact.
Our validated LC-MS/MS workflows deliver ultra-sensitive, multiplex detection of TMAO, TMA, choline, betaine, carnitine, and related metabolites across a wide range of sample types—including plasma, urine, feces, tissues, and microbial cultures.
We help you:
- Quantify key methylamine pathway metabolites with high sensitivity and specificity
- Investigate host-microbiome interactions and microbial metabolic outputs
- Assess pharmacological or dietary interventions with biologically meaningful data
- Customize data reporting to fit clinical, nutritional, or mechanistic research needs
Gain reliable, pathway-focused data to support your research in microbiome science, metabolic health, and precision nutrition.
Submit Your Request Now
×
What You Will Receive
- Quantitative report of TMAO and related metabolites (Excel + PDF)
- Raw LC-MS/MS data files (e.g., .wiff/.d) upon request
- Internal standard-normalized concentration tables
- Chromatograms and peak integration visuals
- Optional: Creatinine normalization, metabolic pathway annotations
- What We Provide
- Advantages
- Technology Platform
- Sample Requirements
- Demo
- FAQs
- Case Study
Overview of TMAO
Trimethylamine N-oxide (TMAO) is a small organic compound derived primarily from dietary nutrients such as choline, L-carnitine, and phosphatidylcholine through gut microbial metabolism and hepatic oxidation. It plays a pivotal role in host-microbiome interactions and has been extensively studied for its involvement in cardiovascular health, kidney function, lipid metabolism, and microbial ecology.
In biological matrices, TMAO functions as an osmolyte, a biomarker of microbial metabolism, and a key indicator of systemic metabolic shifts. Accurate quantification of TMAO and its precursors is thus essential in both mechanistic research and biomarker discovery across preclinical and translational research settings.
Why Analyze TMAO?
Due to its role as a biomarker of gut microbial activity and its association with metabolic health, TMAO analysis is increasingly integrated into:
- Host-microbiome interaction studies
- Nutritional and dietary intervention assessments
- Metabolomic profiling in disease model research
- Pharmaceutical and toxicological compound evaluation
The precise measurement of TMAO levels in plasma, serum, urine, tissue, and fecal samples can uncover critical insights into metabolic pathways and systemic biochemical responses.
TMAO Analysis Service Offered by Creative Proteomics
- Absolute Quantification of TMAO: Accurate measurement of TMAO levels in plasma, urine, tissue, or microbial cultures using isotope-dilution LC-MS/MS.
- Multiplex Analysis of TMAO-Related Metabolites: Simultaneous quantification of choline, carnitine, betaine, TMA, DMG, and γ-butyrobetaine in a single run.
- Microbiota-Derived TMA/TMAO Pathway Profiling: Detection of microbial methylamines to assess gut-origin contributions to systemic TMAO levels.
- Kinetic and Time-Course Analysis: Measurement of TMAO and precursors across multiple timepoints for dynamic pathway analysis.
- Multi-Species Sample Compatibility: Optimized protocols for human, mouse, rat, pig, and zebrafish matrices including plasma and organ extracts.
- Custom Method Development and MRM Transition Optimization: Targeted assay development for specific derivatives or pathway intermediates upon request.
- Stable Isotope Tracing Experiments: ^13C- or D-labeled substrates supported for metabolic flux analysis of TMAO biosynthesis.
- Batch Study and Dose Response Support: Designed for studies comparing dietary, pharmacological, or environmental exposures on TMAO pathways.
- Targeted Pathway Annotation and Data Interpretation (Optional): Annotated reports including metabolite concentrations mapped to related metabolic networks (e.g., KEGG).
Detected TMAO and Related Analytes
| Compound Name | Classification | Related Pathway or Function |
|---|---|---|
| Trimethylamine N-oxide (TMAO) | Main analyte | Hepatic oxidation of TMA; microbial-host co-metabolism |
| Trimethylamine (TMA) | Microbial precursor | Gut microbial metabolism of choline/carnitine |
| Choline | Dietary precursor | Choline metabolism; phospholipid synthesis |
| Betaine (Trimethylglycine) | Methyl donor | One-carbon metabolism; choline oxidation product |
| L-Carnitine | Dietary precursor | Carnitine biosynthesis; fatty acid transport |
| γ-Butyrobetaine | Intermediate | Carnitine biosynthesis; substrate for gut TMA production |
| Dimethylglycine (DMG) | Secondary metabolite | Byproduct of betaine demethylation |
| Sarcosine (N-Methylglycine) | Related methylamine | Intermediate in glycine and methyl group metabolism |
| TMAO-d9 / 13C3-TMAO | Isotope-labeled internal standard | Used for absolute quantification |
| Deuterated Choline (Choline-d9) | Internal standard | Quantitative correction for choline pathway analysis |
| Deuterated Carnitine (Carnitine-d3 or d9) | Internal standard | Used in multiplex carnitine–TMA–TMAO quantification |
| Creatinine | Optional co-analysis | Used for urinary TMAO normalization |
| Acetylcarnitine (C2) | Related metabolite | Reflects carnitine pool and fatty acid oxidation |
| Betaine Aldehyde | Rare intermediate | Short-lived in choline oxidation; detectable with optimization |
| N,N-Dimethylbutylamine | Structural analog | Optional screening in microbial methylamine studies |
| TMA N-oxide derivatives (e.g., hydroxylated TMAO) | Optional | Investigated in oxidative stress and xenobiotic metabolism |
Advantages of TMAO Assay
- High Sensitivity: Quantification limits as low as 0.1 ng/mL in plasma and serum using isotope-dilution LC-MS/MS methods.
- Broad Dynamic Range: Linear detection from 0.1 ng/mL to 10 µg/mL ensures accurate quantification across physiological and pathological concentrations.
- Matrix Versatility: Compatible with plasma, serum, urine, feces, liver, intestine, brain, and microbial culture supernatants.
- Multiplex Capability: Simultaneous quantification of up to 8 related metabolites per run.
- Analytical Precision: Coefficient of variation (CV) < 8% in inter- and intra-batch replicates.
- Custom Data Outputs: Raw data files, peak area reports, concentration tables, and optional pathway visualization.
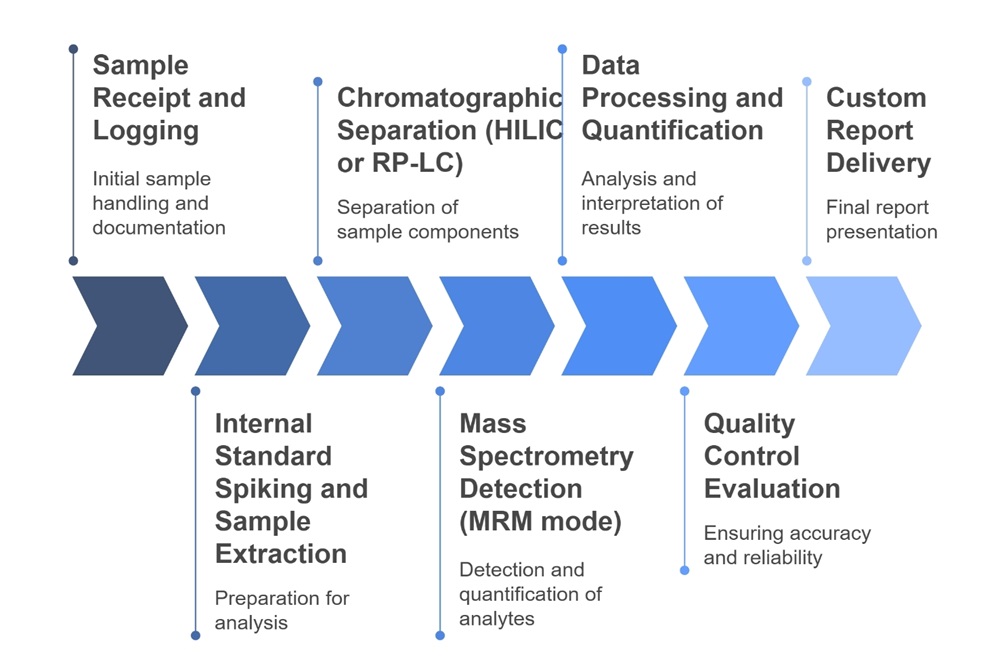
Workflow for TMAO Analysis Service
Technology Platform for TMAO Analysis Service
Agilent 6495C Triple Quadrupole LC-MS/MS
Operated in Multiple Reaction Monitoring (MRM) mode with positive electrospray ionization (ESI+), this system enables precise quantification of TMAO, choline, carnitine, and other methylamines down to sub-ng/mL levels across various biological matrices.
Agilent 1260 Infinity II HPLC
Serves as the front-end chromatographic system, equipped with HILIC or C18 columns to achieve efficient retention and separation of highly polar compounds such as TMAO and its precursors.

Agilent 6495C Triple Quadrupole (Figure from Agilent)

Agilent 1260 Infinity II HPLC (Fig from Agilent)
Sample Requirements for TMAO Analysis Service
| Sample Type | Recommended Amount | Notes |
|---|---|---|
| Plasma | ≥ 100 µL | EDTA or heparin plasma preferred; avoid hemolysis |
| Serum | ≥ 100 µL | Collect after clotting; centrifuge and aliquot promptly |
| Urine | ≥ 200 µL | Midstream or 24-hour urine acceptable; store at −80°C |
| Feces | ≥ 100 mg (wet weight) | Freeze-dried or fresh frozen; avoid contamination |
| Liver Tissue | ≥ 30 mg | Snap-frozen in liquid nitrogen; avoid freeze-thaw cycles |
| Brain Tissue | ≥ 30 mg | Store at −80°C; region-specific sampling recommended |
| Intestinal Content | ≥ 100 mg or 200 µL slurry | Suitable for gut microbial metabolite profiling |
| Microbial Culture | ≥ 1 mL culture supernatant | Centrifuge and filter before shipping; sterile collection required |
| Cell Pellet (Mammalian) | ≥ 1 × 10⁶ cells | Washed and frozen dry; resuspendable in extraction buffer if needed |
| Other Biofluids | Contact us | Custom matrix validation available upon request |
Demo Results
FAQ of TMAO Analysis Service
Can I submit both plasma and fecal samples in the same project?
Yes, we accept mixed sample types in the same batch. Please clearly label each sample and provide the corresponding matrix information during submission.
How should I label and organize samples before shipping?
Use alphanumeric codes (e.g., T01–T50) and include a matching sample list. Avoid handwritten labels that may smear during freezing.
Is normalization to creatinine available for urine samples?
Yes, creatinine levels can be measured for normalization upon request. Please indicate this when submitting your project.
Can I request raw data files in addition to the processed report?
Yes. We provide both processed data (Excel/PDF) and raw instrument files (e.g., .wiff, .d files) upon request for full traceability.
Is the method suitable for low-volume samples, such as mouse plasma?
Yes, our platform is optimized for small-volume matrices; ≥50 µL may be sufficient depending on concentration range.
What column types are used for TMAO retention?
We typically use HILIC or zwitterionic columns to achieve sharp peak resolution for TMAO and related polar compounds.
How do you ensure sample-to-sample reproducibility?
We run pooled QC samples every 10 injections and apply internal standard normalization to correct for batch drift.
Can I include isotope-labeled substrates in my experiment?
Yes, we support 13C-, 15N-, and D-labeled compound tracing. Please contact us in advance for compatibility review.
What is the minimum number of samples required for a project?
There is no strict minimum. However, we recommend at least 6–8 samples per group to support statistical analysis.
Do you offer metabolite ratio analysis (e.g., TMAO/TMA)?
Yes. Relative ratios and fold changes between metabolites can be included in the final report if requested.
Learn about other Q&A.
TMAO Analysis Service Case Study

Title: A highly sensitive liquid chromatography electrospray ionization mass spectrometry method for quantification of TMA, TMAO and creatinine in mouse urine
Journal: MethodsX
Published: 2017
- Background
- Methods
- Results
- Reference
Trimethylamine (TMA) and its hepatic oxidation product, trimethylamine N-oxide (TMAO), are key biomarkers reflecting gut microbial activity and host metabolic capacity, particularly via the FMO3 enzyme. Their accurate quantification in biofluids such as urine is essential for studying host–microbiota interactions, sex- and strain-dependent metabolic differences, and disease-related metabolic disturbances. Existing analytical methods for TMA and TMAO vary in sensitivity and often require complex sample purification steps. This study aimed to develop a robust, highly sensitive LC-MS method for simultaneous quantification of TMA, TMAO, and creatinine in small-volume mouse urine samples.
The authors established a capillary LC–electrospray ionization–mass spectrometry (cap-LC–ESI–MS) method using selective ion monitoring (SIM) for the detection of TMA (after derivatization), TMAO, and creatinine. Key method features included:
- Derivatization of TMA using ethyl bromoacetate to enhance ionization efficiency.
- Chromatographic separation performed on an ACE C18 column with a 5-minute gradient run.
- Internal standards: ^2H9-TMA, ^2H9-TMAO, and acetaminophen were used for accurate quantification and to minimize matrix effects.
- SIM detection: Specific m/z transitions (e.g., 76 for TMAO, 146 for derivatized TMA, 114 for creatinine) enabled sensitive and selective analysis without interference.
- LOD/LOQ reached 28 pg/mL for TMA, 115 pg/mL for TMAO, and 1 ng/mL for creatinine.
Creative Proteomics' Advantage:
At Creative Proteomics, we offer advanced LC-MS/MS services for TMAO profiling that include derivatization workflows, isotope-labeled internal standards, and SIM/MRM-based quantification. We also provide matrix-matched calibration, creatinine normalization, and low-volume sample compatibility
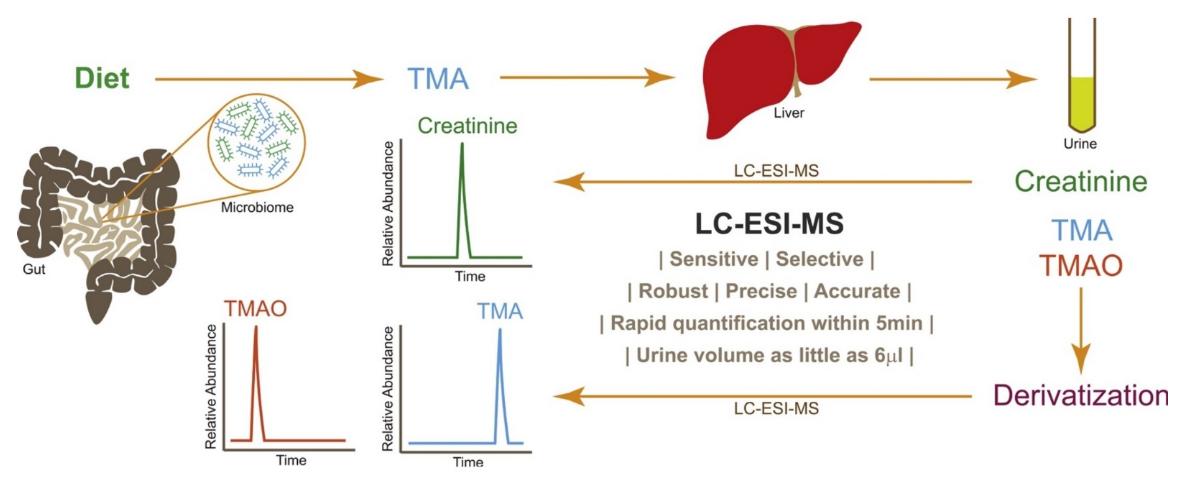 Schematic overview of the LC–ESI–MS workflow for the quantification of TMA, TMAO, and creatinine in mouse urine, incorporating derivatization, C18-based chromatographic separation, and selective ion monitoring (SIM) detection.
Schematic overview of the LC–ESI–MS workflow for the quantification of TMA, TMAO, and creatinine in mouse urine, incorporating derivatization, C18-based chromatographic separation, and selective ion monitoring (SIM) detection.
- The method demonstrated excellent linearity (R² > 0.99) across a broad dynamic range.
- Intra- and inter-day precision were both <6.7%, and recoveries ranged from 96% to 105% for all analytes.
- The platform enabled quantification of all three metabolites from as little as 6 µL of urine, with no need for sample clean-up.
- Sex- and strain-specific differences in urinary TMA/TMAO ratios were confirmed, aligning with known hepatic FMO3 expression patterns.
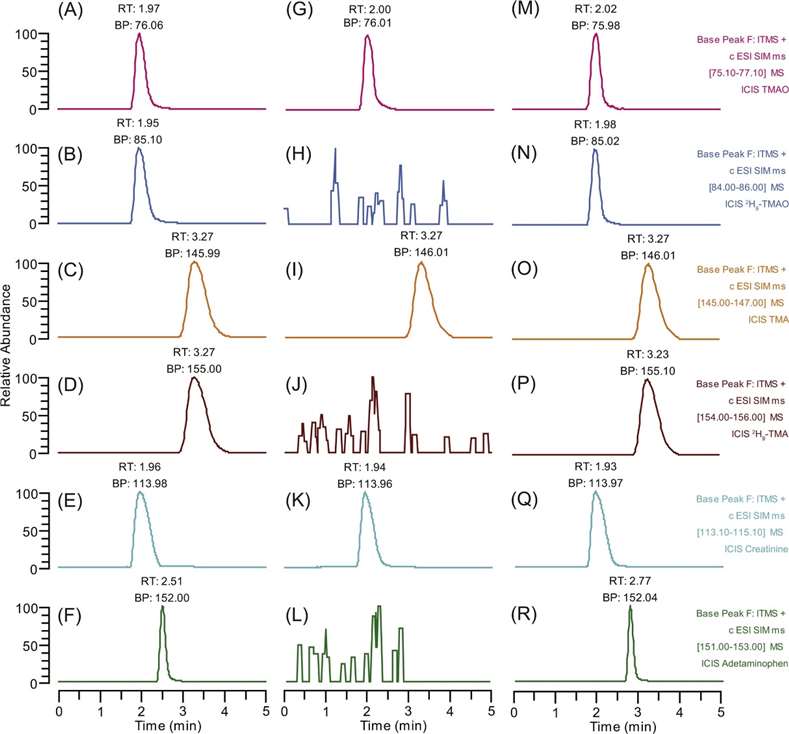 Reconstructed ion chromatograms (RICs) showing SIM detection of TMAO, derivatized TMA, creatinine, and their internal standards in pure standards, mouse urine, and spiked urine samples.
Reconstructed ion chromatograms (RICs) showing SIM detection of TMAO, derivatized TMA, creatinine, and their internal standards in pure standards, mouse urine, and spiked urine samples.
Reference
- Veeravalli, Sunil, et al. "A highly sensitive liquid chromatography electrospray ionization mass spectrometry method for quantification of TMA, TMAO and creatinine in mouse urine." MethodsX 4 (2017): 310-319. https://doi.org/10.1016/j.mex.2017.09.004
Publications
Here are some of the metabolomics-related papers published by our clients:

- White matter lipid alterations during aging in the rhesus monkey brain. 2024. https://doi.org/10.1007/s11357-024-01353-3
- Multiomics of a rice population identifies genes and genomic regions that bestow low glycemic index and high protein content. 2024. https://doi.org/10.1073/pnas.2410598121
- The Brain Metabolome Is Modified by Obesity in a Sex-Dependent Manner. 2024. https://doi.org/10.3390/ijms25063475
- Characterization of Dnajc12 knockout mice, a model of hypodopaminergia. 2024. https://doi.org/10.1101/2024.07.06.602343
- Pan-lysyl oxidase inhibition disrupts fibroinflammatory tumor stroma, rendering cholangiocarcinoma susceptible to chemotherapy. 2024. https://doi.org/10.1097/HC9.0000000000000502
- Glycine supplementation can partially restore oxidative stress-associated glutathione deficiency in ageing cats. 2024. https://doi.org/10.1017/S0007114524000370
- The activity of the aryl hydrocarbon receptor in T cells tunes the gut microenvironment to sustain autoimmunity and neuroinflammation. 2023. https://doi.org/10.1371/journal.pbio.3002000
- Lipin-1 regulates lipid catabolism in pro-resolving macrophages. 2020. https://doi.org/10.1101/2020.06.03.121293
- Characterization of a novel AraC/XylS-regulated family of N-acyltransferases in pathogens of the order Enterobacterales. 2020. https://doi.org/10.1371/journal.ppat.1008776












