Introduction
Tryptophan (Trp) is the largest aromatic amino acid by molecular weight, and its metabolites serve as precursors for the synthesis of various bioactive compounds. As tryptophan cannot be synthesized by the human body, it must be entirely obtained through dietary intake[1]. Once ingested from natural food sources, tryptophan is absorbed into the bloodstream through the intestinal epithelium. Most of it binds to albumin in the blood, while a small portion remains in free form[2]. Tryptophan and its downstream metabolites are involved in human metabolism and immune responses, and their metabolic processes are closely associated with the gut microbiota. The gut microbial community mediates certain pathways of tryptophan metabolism and also influences the functions and effects of its downstream molecules[3].
This article explores the key pathways, analytical techniques, and representative case studies that shed light on gut tryptophan metabolism and its broader biological significance.
Three Principal Tryptophan Metabolic Pathways
In the human body, tryptophan is primarily metabolized through three major pathways: (1) Via indoleamine 2,3-dioxygenase 1 (IDO1), tryptophan is converted into kynurenine (Kyn), which can be further metabolized by 3-hydroxyanthranilate 3,4-dioxygenase (HAAO) into quinolinic acid, a neurotoxic compound; (2) Through tryptophan hydroxylase (TPH), it is converted into 5-hydroxytryptamine (5-HT), commonly known as serotonin; (3) Via microbial metabolism, tryptophan is transformed into indole and related derivatives (Figure 1)[4-6].
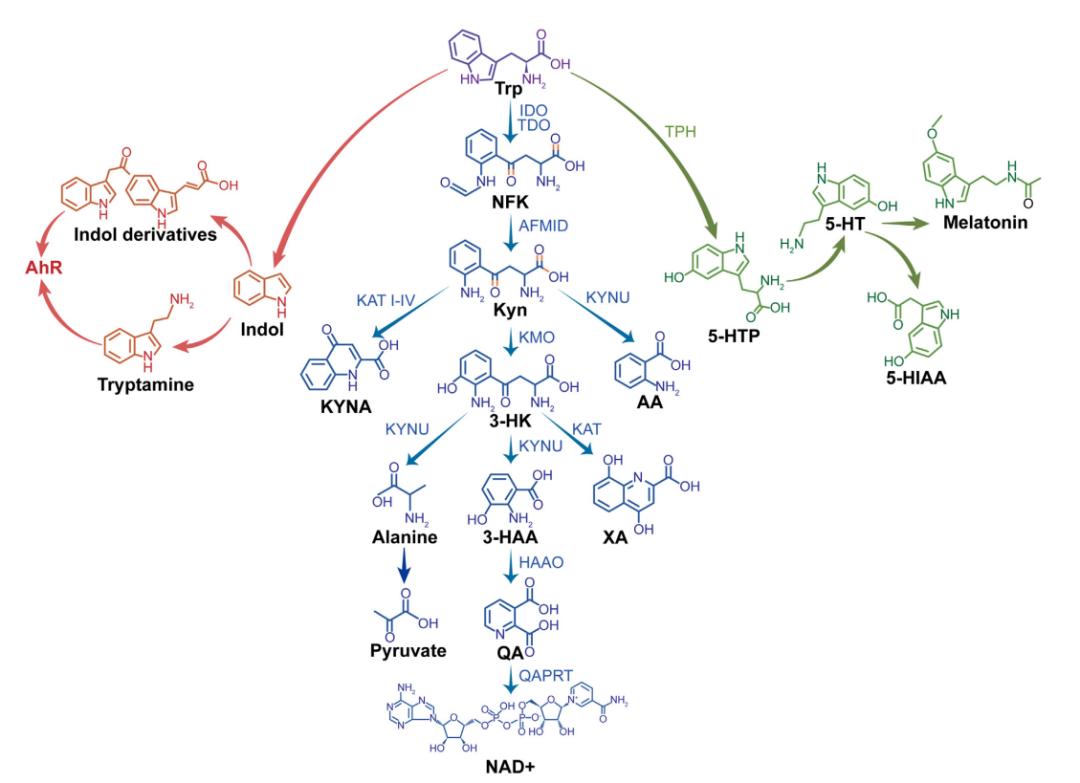 Figure 1 Overview of tryptophan metabolism via the Kyn, 5-HT, and indole pathways
Figure 1 Overview of tryptophan metabolism via the Kyn, 5-HT, and indole pathways
Common Methodologies for Studying Gut Tryptophan Metabolism
Research on gut tryptophan metabolism has rapidly evolved, driven by advances in high-throughput analytical technologies. Among the most commonly employed methodologies are metabolomics analysis, mass spectrometry (MS), and microbiome sequencing, each offering complementary insights into the metabolic landscape shaped by host–microbiota interactions.
Metabolomics, particularly when applied to serum, fecal, or tissue samples, enables comprehensive profiling of tryptophan and its downstream metabolites. Untargeted metabolomics is used to discover novel metabolites or altered metabolic pathways, while targeted metabolomics quantifies specific tryptophan catabolites with high precision[7].
Mass spectrometry, often coupled with liquid chromatography (LC-MS), is a central analytical tool in metabolomics for its sensitivity and ability to resolve structurally similar compounds. It plays a critical role in identifying and quantifying key metabolites along the kynurenine, serotonin, and indole pathways[8].
Meanwhile, 16S rRNA gene sequencing and shotgun metagenomics are routinely used to characterize the gut microbiota, elucidating microbial taxa involved in tryptophan transformation[9]. When combined with metabolomic data, these approaches allow researchers to correlate microbial composition and function with specific metabolic outputs, offering a systems-level understanding of gut–tryptophan interactions.
Select Service
Applications of Mass Spectrometry-Based Metabolomics in Tryptophan Metabolism Research
Mass spectrometry-based metabolomics has become an indispensable tool for dissecting the complex metabolic pathways of tryptophan in the gut. By enabling high-resolution detection and quantification of tryptophan and its derivatives, MS facilitates the study of flux through key metabolic branches, including the kynurenine pathway, the serotonin pathway, and microbially derived indoles.
In targeted approaches, stable isotope-labeled tryptophan is often used in tracer experiments to monitor the dynamic conversion of tryptophan into downstream metabolites under different physiological or microbial conditions[10]. These techniques are particularly valuable for studying how microbial taxa or host enzymes modulate specific branches of tryptophan metabolism.
Additionally, MS-based metabolomics allows for the comparative analysis of metabolic profiles in health and disease, revealing alterations in tryptophan metabolism associated with conditions such as inflammatory bowel disease, depression, or metabolic disorders[11, 12]. Through integration with microbiome sequencing and transcriptomics, mass spectrometry data further enables multi-omics correlation analyses, offering mechanistic insights into host–microbiota interactions and their impact on immune and neuroactive pathways governed by tryptophan metabolites.
Select Service
Comparative Analysis of Methodologies in Gut Tryptophan Metabolism Research
To comprehensively investigate gut tryptophan metabolism, researchers commonly employ a combination of metabolomics, mass spectrometry (MS), and microbiome sequencing. Each method has distinct advantages and limitations in terms of precision, sensitivity, applicability, and cost.
Metabolomics offers a global view of metabolic alterations and is particularly useful in profiling tryptophan-related pathways. Untargeted approaches allow the discovery of novel metabolic signatures, while targeted metabolomics enables quantification of specific metabolites. However, its performance depends heavily on the analytical platform and proper data interpretation.
Mass spectrometry-based analysis, especially LC-MS/MS, remains the gold standard for quantifying tryptophan and its derivatives with high precision and sensitivity. It supports detailed mechanistic studies, including isotopic tracer experiments and dynamic flux analysis, but requires complex sample preparation and access to high-end instrumentation.
Microbiome sequencing, including 16S rRNA gene sequencing and shotgun metagenomics, provides critical information about microbial taxa and functional genes involved in tryptophan transformation. While it does not directly measure metabolites, it enables inference of microbial contributions to tryptophan metabolism and is indispensable in microbiota–host interaction studies.
The choice of method depends on the specific research question (Table 1). For broad metabolic profiling or biomarker discovery, metabolomics is most suitable. For mechanistic validation and targeted quantification, MS is preferred. For assessing microbial contributions, microbiome sequencing is essential. Increasingly, multi-omics integration is employed to achieve a systems-level understanding of the gut–microbiota–tryptophan axis.
Table1: Comparison of Common Methods in Gut Tryptophan Metabolism Research
| Method | Platform | Precision | Sensitivity | Main Advantages | Limitations | Typical Applications |
|---|---|---|---|---|---|---|
| Metabolomics | LC-MS / GC-MS / NMR | Medium–High | High | Broad metabolic coverage; pathway discovery | Dependent on instrumentation; limited for very low-abundance metabolites | Discovery of metabolic signatures; pathway mapping; biomarker studies |
| Mass Spectrometry (MS) | LC-MS/MS (targeted) | High | Very High | High-resolution quantification; suitable for tracer studies | High cost; requires technical expertise | Quantification of specific metabolites; isotope tracing; mechanistic studies |
| Microbiome Sequencing | 16S rRNA / Shotgun WGS | Medium | High | Identifies microbial taxa and functional genes | Indirect inference of metabolic function; no metabolite data | Microbiota profiling; microbial function inference; host–microbe interaction studies |
Case Study of Gut MicrobiotaTryptophan Metabolism
Application of Metabomics Analysis in Gut Tryptophan Metabolism Research
In the study by Chojnowski et al. (2025)[13], untargeted metabolomics was employed to investigate metabolic alterations in pediatric epilepsy, with a particular focus on tryptophan metabolism and the gut–brain axis. Plasma samples from 18 pediatric epilepsy patients and 11 age-matched healthy controls were analyzed using liquid chromatography–mass spectrometry (LC-MS). The data underwent both univariate and multivariate statistical analyses, along with pathway enrichment analysis. Multivariate analyses revealed a clear separation between patient and control groups. Nineteen endogenous metabolites (VIP > 1, adjusted p < 0.05) were identified as key differentiators (Figure 2).
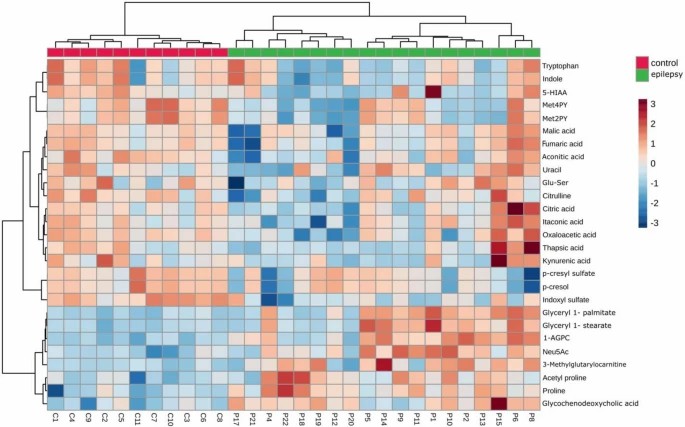 Figure 2. Hierarchical Clustering analysis of all important metabolites (adj. p-value < 0.05, VIP > 1) represented as a heatmap, generated using Euclidean distance and Ward’s clustering algorithm.
Figure 2. Hierarchical Clustering analysis of all important metabolites (adj. p-value < 0.05, VIP > 1) represented as a heatmap, generated using Euclidean distance and Ward’s clustering algorithm.
Compared to controls, epilepsy patients exhibited significant reductions in tryptophan (Trp), 5-hydroxyindoleacetic acid (5-HIAA), several gut microbiota-derived metabolites—including indole, indoxyl sulfate, and p-cresyl sulfate—as well as niacin metabolism end-products such as N1-methyl-2-pyridone-5-carboxamide (Met2PY) and N1-methyl-4-pyridone-3-carboxamide (Met4PY). Additionally, patients showed decreased levels of tricarboxylic acid (TCA) cycle intermediates, alongside increased levels of fatty acid derivatives and N-acetylneuraminic acid (Neu5Ac). The most substantially altered metabolic pathways in epilepsy patients involved the TCA cycle, vitamin A and C metabolism, prostaglandin synthesis, and D4/E4-neuroprostane formation.
These findings suggest that gut dysbiosis may contribute to epilepsy development through the gut–brain axis. Furthermore, the observed metabolic markers indicating energy deficits and oxidative stress underscore the systemic impact of seizure activity. This study highlights the potential of untargeted metabolomics in uncovering metabolic disturbances associated with pediatric epilepsy and underscores the importance of considering gut microbiota and tryptophan metabolism in understanding the disease's pathophysiology.
Conclusion
The study of gut tryptophan metabolism relies on cutting-edge methodologies that provide crucial insights into host-microbiota interactions. Metabolomics analysis, particularly mass spectrometry (MS)-based approaches, enables precise quantification of tryptophan derivatives along key pathways like kynurenine and serotonin, revealing metabolic dysregulation in diseases. Meanwhile, 16S rRNA sequencing identifies microbial taxa involved in tryptophan transformation, linking microbial composition to metabolic outputs. Their integration through multi-omics approaches offers systems-level understanding of gut metabolic networks. Future advancements could focus on:
Spatiotemporal resolution – Developing single-cell metabolomics and in situ imaging to map metabolite-microbe interactions spatially[15]; Dynamic modeling – Combining isotope tracing with AI to predict metabolic flux variations; Functional validation – Using gnotobiotic models to test causality in microbial-metabolite relationships. The comprehensive application of these technologies and research methods will help enhance the integrity of research and precisely analyze the dynamic changes of metabolites and their associations with diseases.
References
- EVENEPOEL P, CLAUS D, GEYPENS B, et al. Amount and fate of egg protein escaping assimilation in the small intestine of humans. Am J Physiol, 1999, 277(5): G935-43. DOI: 10.1152/ajpgi.1999.277.5.G935
- GIBSON J A, SLADEN G E, DAWSON A M. Protein absorption and ammonia production: the effects of dietary protein and removal of the colon. Br J Nutr, 1976, 35(1): 61-5. DOI: https://doi.org/10.1079/BJN19760009
- ROAGER H M, HANSEN L B, BAHL M I, et al. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat Microbiol, 2016, 1(9): 16093. https://doi.org/10.1038/nmicrobiol.2016.93
- XUE C, LI G, ZHENG Q, et al. Tryptophan metabolism in health and disease. Cell Metab, 2023, 35(8): 1304-26. DOI: 10.1016/j.cmet.2023.06.004
- DAUBERT E A, CONDRON B G. Serotonin: a regulator of neuronal morphology and circuitry. Trends Neurosci, 2010, 33(9): 424-34. DOI: 10.1016/j.tins.2010.05.005
- WEI G Z, MARTIN K A, XING P Y, et al. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A, 2021, 118(27. DOI: 10.1073/pnas.2021091118
- VICH VILA A, ZHANG J, LIU M, et al. Untargeted faecal metabolomics for the discovery of biomarkers and treatment targets for inflammatory bowel diseases. Gut, 2024, 73(11): 1909-20. DOI: 10.1136/gutjnl-2023-329969
- VAN LAARHOVEN A, DIAN S, AGUIRRE-GAMBOA R, et al. Cerebral tryptophan metabolism and outcome of tuberculous meningitis: an observational cohort study. Lancet Infect Dis, 2018, 18(5): 526-35. DOI: 10.1016/S1473-3099(18)30053-7
- LAMAS B, RICHARD M L, LEDUCQ V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med, 2016, 22(6): 598-605. DOI: 10.1038/nm.4102
- GEHIN M, WELFORD R W D, GARZOTTI M, et al. Assessment of Peripheral Serotonin Synthesis Using Stable Isotope-Labeled Tryptophan. Clin Pharmacol Ther, 2018, 104(6): 1260-7. DOI: 10.1002/cpt.1087
- WIKOFF W R, ANFORA A T, LIU J, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A, 2009, 106(10): 3698-703. https://doi.org/10.1073/pnas.0812874106
- LI Y, YANG X, CHEN S, et al. Integrated Network Pharmacology and GC-MS-Based Metabolomics to Investigate the Effect of Xiang-Su Volatile Oil Against Menopausal Depression. Front Pharmacol, 2021, 12: 765638. DOI: 10.3389/fphar.2021.765638
- Chojnowski, K., Opiełka, M., Urbanowicz, K. et al. Untargeted metabolomics reveals key metabolic alterations in pediatric epilepsy with insights into Tryptophan metabolism and the gut–brain axis. Sci Rep 15, 15262 (2025). https://doi.org/10.1038/s41598-025-99805-z
- ZHOU B, JIN G, PANG X, et al. Lactobacillus rhamnosus GG colonization in early life regulates gut-brain axis and relieves anxiety-like behavior in adulthood. Pharmacol Res, 2022, 177: 106090. DOI: 10.1016/j.phrs.2022.106090
- SEYDEL C. Single-cell metabolomics hits its stride. Nat Methods, 2021, 18(12): 1452-6. https://doi.org/10.1038/s41592-021-01333-x






