L-carnitine represents a small molecular weight amino acid derivative synthesized through metabolic pathways involving two indispensable amino acids: methionine and lysine. This compound serves an essential function in lipid metabolism, particularly facilitating the transport of long-chain fatty acid molecules across the mitochondrial membrane into the matrix, where β-oxidation occurs. The biosynthetic process enables cells to efficiently utilize fatty acids as an energy source through this critical transport mechanism.
What is Carnitine?
Carnitine is an amino acid and a quaternary ammonium cation complex. It can be synthesized from two amino acids, lysine and methionine, through biosynthesis. It is related to the metabolism of fat into energy in the body. It was discovered and extracted from muscle in 1905. Its chemical formula is (CH3)3N+ CH2CH(OH)CH2COO-.
L-Carnitine (β-hydroxy-γ-trimethylaminobutyric acid), also known as vitamin BT and carnitine, is a biologically active low molecular weight (161.5 g/mol) amino acid derived from the essential amino acids L-lysine and L-methionine. Its optical isomer D-carnitine has no biological activity and cannot be produced by the body. Under physiological pH conditions, L-carnitine is a zwitterionic ion. It contains a positively charged quaternary ammonium and a negatively charged carboxyl group, connected by a three-carbon short chain. One carbon of this three-carbon short chain is connected to a hydroxyl group, which can combine with organic acids and fatty acids to form lipids of different chain lengths. The transfer of active acyl groups between carnitine and acetyl-CoA is the biologically active center of carnitine. This acyl transfer is catalyzed by many long-chain specific carnitine acyltransferases.
Endogenous Synthesis and Sources of Carnitine
L-carnitine demonstrates ubiquitous distribution across cellular environments, being present in virtually all mammalian tissues, plant systems, and microbial organisms. Animal species acquire this compound through two primary mechanisms: dietary consumption and internal biosynthetic processes.
Within human physiology, approximately three-quarters of total carnitine originates from nutritional sources, while the remaining quarter derives from endogenous metabolic pathways. Intestinal absorption occurs predominantly in the small bowel through active transport systems, specifically mediated by organic cation transporters (OCTN family).
Hepatic tissue serves as the principal site for carnitine biosynthesis, although secondary synthesis also occurs in renal, epididymal, and cerebral tissues. The kidneys function as the primary regulatory organ for maintaining systemic carnitine homeostasis and controlling elimination processes. Renal tubular reabsorption is remarkably efficient, with over ninety percent of filtered carnitine being reclaimed, resulting in minimal urinary losses under normal physiological conditions.
Endogenous synthesis from lysine and methionine
Carnitine is mainly synthesized in the human body through endogenous pathways, a process that mainly occurs in the liver and kidneys. Its synthesis depends on two essential amino acids: lysine and methionine. Lysine provides the carbon skeleton of the carnitine molecule, while methionine provides the methyl group required for N-methylation.
The synthesis process includes multiple steps:
Lysine residues are methylated to form ε-N-trimethyl-lysine (TML), which requires vitamin C, vitamin B6, niacin and iron as cofactors. TML undergoes a series of enzymatic reactions, including ε-N-trimethyl-lysine hydroxylase (HTMLA), γ-butyryl transferase (BBOX), etc., and is finally converted into L-carnitine. In addition, skeletal muscle and cardiac muscle cells cannot synthesize carnitine on their own, so they are completely dependent on the supply of carnitine in the blood.
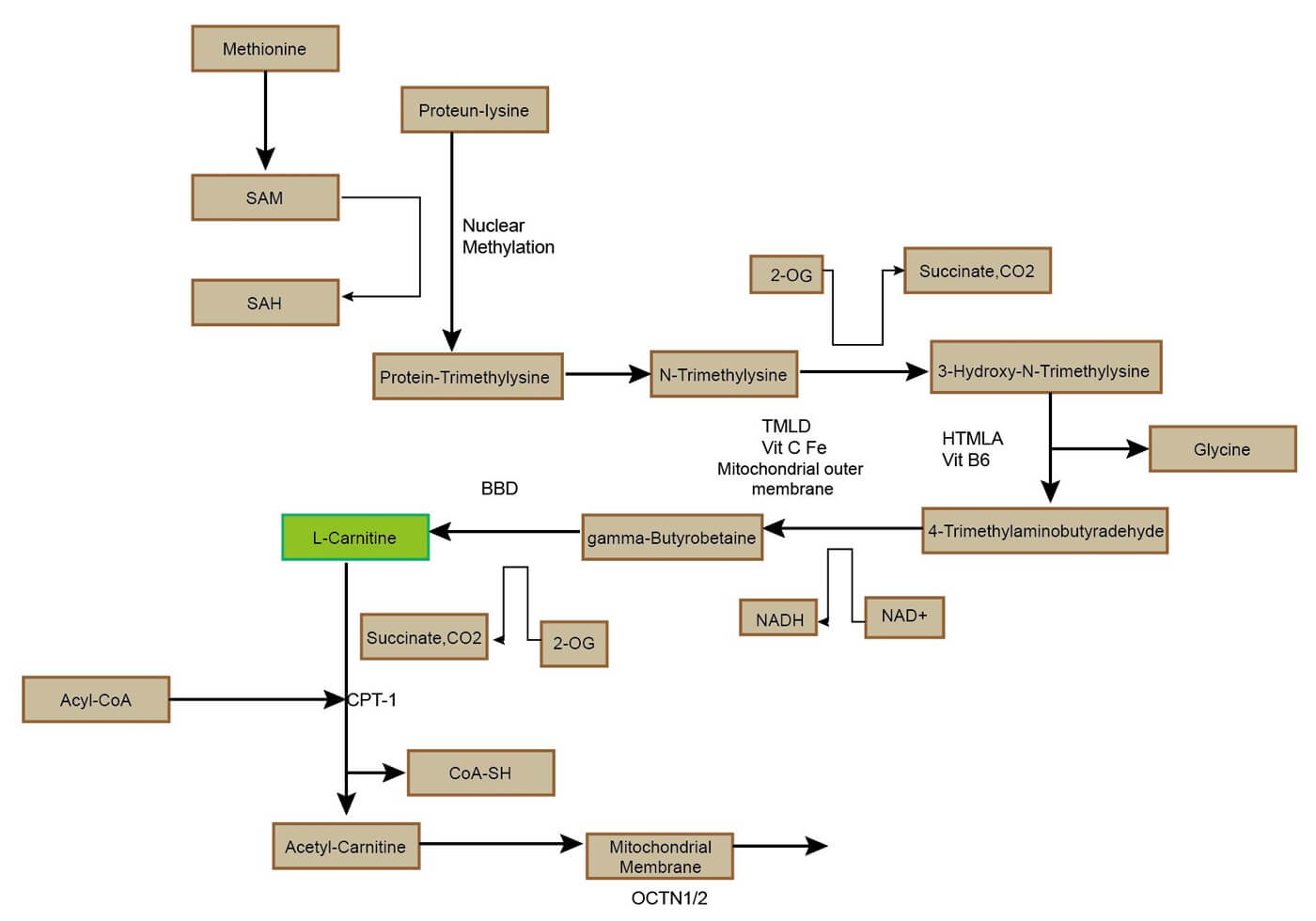 Figure 1. The metabolic pathways of carnitine.
Figure 1. The metabolic pathways of carnitine.
Carnitine transmembrane transport
Carnitine transport in and out of cells depends on specific transporters, among which OCTN2 transporter plays a key role. OCTN2 is a sodium-dependent high-affinity carnitine transporter widely expressed in kidney, skeletal muscle, heart and other tissues.
Carnitine transport in vivo is regulated by a dedicated network of membrane transporters showing different tissue and subcellular localizations. Intestinal absorption of carnitine and its renal reabsorption are mainly mediated by the plasma membrane organic cation transporter 2 (OCTN2–SLC22A5), which has the highest affinity for carnitine among the carnitine handling transporters. OCTN2 also plays an important role in the distribution of carnitine to various tissues. Carnitine transport mediated by OCTN2 is Na+ dependent. This property allows carnitine to accumulate in cells, creating a concentration gradient between the intracellular space and the blood. The intracellular concentration of carnitine ranges from 1 to 5 mM, while the concentration in plasma ranges from 25 to 50 μM.
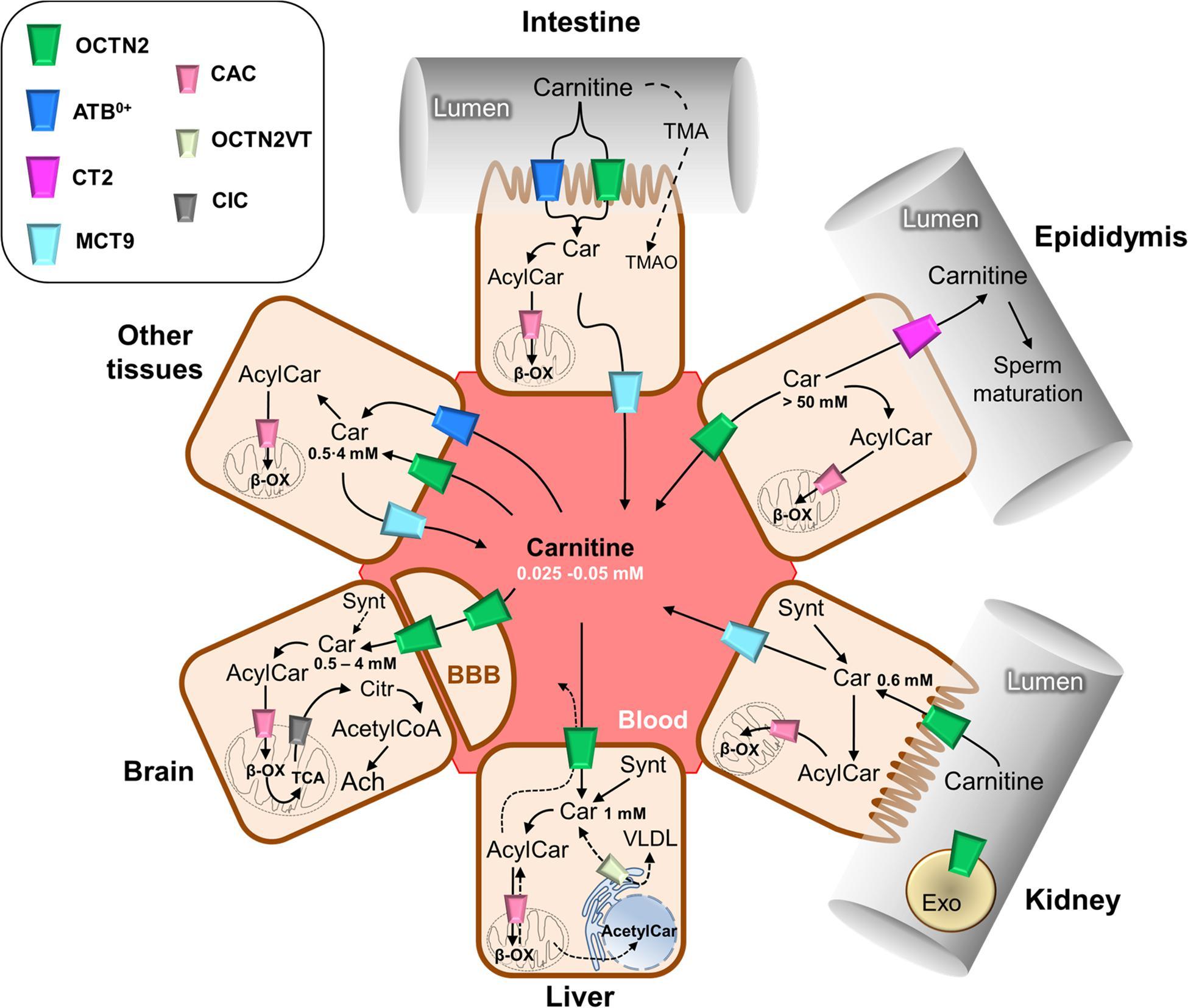 Figure 2. Schematic representation of carnitine traffic (Console, L. et al. 2020).
Figure 2. Schematic representation of carnitine traffic (Console, L. et al. 2020).
The biosynthesis of carnitine depends on lysine and methionine, and requires a variety of vitamins and minerals as cofactors; its dietary sources are mainly concentrated in animal foods; and the OCTN2 transporter is responsible for the absorption and transportation of carnitine in the body. These mechanisms together maintain the steady-state level of carnitine in the human body, thereby supporting fatty acid oxidation and energy metabolism.
Carnitine Shuttle and Fatty Acid β-Oxidation
Carnitine plays a vital role in fatty acid β-oxidation and energy metabolism. Its main function is to transport long-chain fatty acids (LCFA) from the cytoplasm to the mitochondrial matrix through the carnitine shuttle system, thereby entering the β-oxidation process.
Key enzymes in the carnitine transport system
- Carnitine Palmitoyltransferase I (CPT I): Located on the outer mitochondrial membrane, CPT I catalyzes the formation of acyl-carnitine from long-chain acyl-CoA and carnitine. This is the first step in the transport of fatty acids into the mitochondria.
- Carnitine-Acylcarnitine Translocase (CACT): Situated in the inner mitochondrial membrane, CACT is responsible for the transport of acyl-carnitine from the intermembrane space into the mitochondrial matrix.
- Carnitine Palmitoyltransferase II (CPT II): Located on the inner mitochondrial membrane, CPT II catalyzes the conversion of acyl-carnitine back to acyl-CoA and free carnitine. This step regenerates carnitine, which can then be transported back to the cytosol.
Steps of the carnitine shuttle system
Formation of Acyl-Carnitine: Long-chain fatty acids in the cytosol are activated to acyl-CoA, which then reacts with carnitine in the presence of CPT I to form acyl-carnitine.
Transport into the Mitochondrial Matrix: Acyl-carnitine is transported across the inner mitochondrial membrane by CACT.
Regeneration of Carnitine: Once inside the mitochondrial matrix, acyl-carnitine is converted back to acyl-CoA and free carnitine by CPT II. The free carnitine is then transported back to the cytosol via CACT, completing the cycle.
β-Oxidation process
Breakdown of Acyl-CoA: The acyl-CoA generated from the carnitine shuttle enters the mitochondrial matrix and undergoes β-oxidation. This process involves the sequential removal of two-carbon units from the fatty acid chain, producing acetyl-CoA, FADH2, and NADH.
Energy Production: The acetyl-CoA produced enters the citric acid cycle (TCA cycle), while FADH2 and NADH are used in the electron transport chain to generate ATP through oxidative phosphorylation. This process ultimately provides energy for cellular functions.
The carnitine shuttle system is essential for the transport of long-chain fatty acids into the mitochondria for β-oxidation. Key enzymes such as CPT I, CACT, and CPT II facilitate the formation, transport, and regeneration of acyl-carnitine, ensuring the efficient breakdown of fatty acids into energy. This process is critical for maintaining cellular energy homeostasis and overall metabolic function.
Disorders Associated with Carnitine Metabolism
Diseases related to carnitine metabolism include primary carnitine deficiency (PCD), secondary carnitine deficiency (such as diabetes, kidney disease, etc.), and congenital defects (such as CPT II deficiency, CACT deficiency, etc.). The symptoms and clinical effects of these diseases vary depending on the type and individual differences.
Primary carnitine deficiency
PCD is a rare genetic disease caused by mutations in the SLC22A5 gene, which leads to dysfunction of the carnitine transporter OCTN2, thereby hindering the entry of carnitine from the blood into cells and the reabsorption of carnitine by the kidneys. The symptoms of PCD range widely, from metabolic decompensation in infancy to asymptomatic manifestations in adulthood. Metabolic decompensation: usually occurs in infants aged 3 to 24 months, manifested by hypoglycemic coma, feeding difficulties, irritability, lethargy, hepatomegaly, elevated liver enzymes, and elevated transaminases. Muscle and heart disease: including muscle weakness, cardiomyopathy (myocardial hypertrophy or dilation), and arrhythmias.Nervous system effects: may manifest as brain damage, seizures, intellectual disability, ataxia, etc. Other symptoms: including fatigue, vomiting, abdominal pain, growth retardation, weight loss, etc.
Secondary carnitine deficiency
Secondary carnitine deficiency is a condition characterized by reduced carnitine levels resulting from various underlying diseases or conditions. Common causes encompass metabolic disorders like fatty acid oxidation defects and organic acidemias, renal issues such as carnitine loss due to chronic renal failure, Fanconi syndrome, dialysis, or certain medications (e.g., vancomycin), dietary factors including vegetarianism, starvation, or specific diets like the ketogenic diet, and liver diseases such as cirrhosis and fatty liver. Symptoms may manifest as muscle weakness, cardiomyopathy, hypoglycemic coma, and abnormal liver function. Treatment typically involves addressing the underlying cause and may include L-carnitine supplementation to alleviate symptoms.
Inborn errors
Congenital defects involve enzyme deficiencies in the carnitine transport or metabolic pathways, such as CPT II deficiency, which affects the function of the carnitine transporter CPT II and leads to impaired fatty acid β-oxidation, and CACT deficiency, which affects the function of the carnitine transporter CACT and results in carnitine shuttle dysfunction. These defects typically manifest as severe metabolic disorders, such as cardiomyopathy, encephalopathy, and hypoglycemia.
The relationship between carnitine and cancer
Cancer cells exhibit metabolic flexibility, utilizing various substrates for energy production to support rapid proliferation. While glucose and glutamine are primary energy sources, some cancer types also rely on FAO. This metabolic heterogeneity suggests that cancer cells can adapt their metabolism based on the availability of substrates and the tumor microenvironment.
Some tumors, particularly those with high energy demands, utilize FAO as a primary energy source. Carnitine is essential for this process, as it shuttles fatty acids into the mitochondria for β-oxidation. Different cancer types and stages exhibit varying reliance on FAO, indicating that carnitine's role may differ based on the specific metabolic needs of the tumor.
Given the role of carnitine transporters in cancer metabolism, targeting these transporters could disrupt FAO and potentially inhibit tumor growth.Carnitine transporters can be exploited for targeted drug delivery, enhancing the efficacy of chemotherapy.Expression levels of carnitine transporters like OCTN2 and CT2 could serve as prognostic markers for certain cancers.
Analytical Techniques for Carnitine and Acylcarnitines
Analytical techniques play a key role in the quantitative analysis of carnitine and acylcarnitine, especially in metabolomics studies. Liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based quantitative methods are one of the most commonly used techniques, with the advantage of being able to efficiently separate and detect a variety of carnitine and its derivatives, including free carnitine, acylcarnitine, and their isomers and odd forms. These techniques are widely used in metabolite analysis of plasma, urine, and tissue samples, and also play an important role in biomarker discovery.
LC-MS/MS basic quantitative method
LC-MS/MS is a technology that combines the separation power of liquid chromatography and the sensitivity of mass spectrometry detection, and is suitable for the quantitative analysis of carnitine and its derivatives. By using stable isotope-labeled internal standards, high sensitivity and high specificity detection of carnitine and its acyl derivatives can be achieved. For example, the LC-MS/MS method mentioned in the study can simultaneously quantify up to 30 carnitines and their analogs, covering the range of C0 to C18, and significantly improves the accuracy and throughput of quantitative analysis.
Targeted metabolomics methods
Targeted metabolomics methods focus on the quantitative analysis of known metabolites, which makes them valuable in disease diagnosis and biomarker discovery. For example, through LC-MS/MS technology, researchers are able to quantify amino acids and acylcarnitines in plasma, which are considered potential biomarkers of metabolic syndrome. In addition, this method has also been used in biomarker research for diseases such as diabetes and obesity.
Sample preparation
Sample preparation is a key step in LC-MS/MS analysis, including methods such as protein precipitation, liquid-liquid extraction, and solid phase extraction. These techniques help reduce interfering substances in the sample and improve the sensitivity and accuracy of the analysis. For example, in plasma and tissue samples, samples are usually derivatized by dissolving in methanol and adding internal standards to improve the sensitivity and stability of the detection.
Research and Clinical Applications
Role of carnitine in metabolic diseases
Carnitine is a conditionally essential amino acid derivative that plays a key role in fatty acid oxidation and energy metabolism. It promotes energy production by transporting long-chain fatty acids to the mitochondrial matrix for β-oxidation. Studies have shown that carnitine deficiency is associated with a variety of metabolic diseases, including diabetes, obesity, cardiovascular disease, and chronic kidney disease. In addition, abnormal carnitine levels may also affect carbohydrate metabolism. For example, in diabetic patients, carnitine abnormalities may lead to insulin resistance and glycemic control problems.
Role of carnitine in cardiovascular diseases
Carnitine has potential benefits for cardiovascular health. Studies have shown that carnitine supplementation can improve symptoms of myocardial ischemia, heart failure, and coronary artery disease, and improve exercise tolerance. In addition, carnitine may have a therapeutic effect on chronic cardiomyopathy and acute ischemic syndrome by enhancing mitochondrial function and reducing oxidative stress. However, some studies have also pointed out that carnitine has limited preventive effects on cardiovascular disease.
Role of carnitine in obesity and diabetes
Carnitine plays an important role in regulating energy metabolism and improving insulin sensitivity. In patients with obesity and type 2 diabetes, carnitine supplementation improves insulin-induced glucose utilization and increases fatty acid oxidation rates. In addition, carnitine may be beneficial for weight management by promoting β-oxidation of fatty acids, which helps reduce body fat accumulation. However, there is still controversy about the effect of carnitine in the treatment of diabetes, and some studies have pointed out that it may increase certain cardiovascular risks.
Application of carnitine supplementation in treatment and sports nutrition
Carnitine supplementation is widely used to treat carnitine deficiency and prevent diseases related to metabolic disorders. In the field of sports nutrition, carnitine is believed to improve muscle endurance and reduce fatigue. However, despite a large number of studies supporting the potential benefits of carnitine on athletic performance, there is insufficient evidence for its effects in healthy individuals.
Translational insights from carnitine metabolism studies
In recent years, the development of mass spectrometry has enabled researchers to more accurately measure the levels of carnitine and its esters (such as acetylcarnitine), revealing the connection between carnitine homeostasis and metabolic diseases. These studies have provided important clues to understand the mechanism of action of carnitine in different pathological states and laid the foundation for the development of new therapeutic strategies.
Conclusion
L-carnitine represents a quaternary ammonium compound with critical roles in cellular bioenergetics, particularly facilitating β-oxidation processes involving fatty acids. This biomolecule enhances lipid oxidation for energy production through its capacity to shuttle long-chain acyl moieties across mitochondrial membranes from cytoplasmic compartments into the matrix. Furthermore, L-carnitine maintains optimal acyl-CoA to free CoA ratios within mitochondria, consequently modulating carbohydrate and protein metabolic processes.
Endogenous carnitine production occurs predominantly via biosynthetic cascades utilizing lysine and methionine as precursor substrates. The synthetic pathway encompasses numerous enzyme-mediated transformations, including a crucial reaction catalyzed by γ-butyrobetaine hydroxylase (γ-BBH). Physiological carnitine balance requires coordinated integration of three fundamental components: nutritional acquisition, internal biosynthesis, and renal tubular conservation mechanisms. Inadequate carnitine availability can precipitate significant metabolic disturbances and associated pathological conditions.
References
- Console, L., Scalise, M., Mazza, T., Pochini, L., Galluccio, M., Giangregorio, N., Tonazzi, A., & Indiveri, C. (2020). Carnitine Traffic in Cells. Link With Cancer. Frontiers in cell and developmental biology, 8, 583850. https://doi.org/10.3389/fcell.2020.583850
- Elantary, R., & Othman, S. (2024). Role of L-carnitine in Cardiovascular Health: Literature Review. Cureus, 16(9), e70279. https://doi.org/10.7759/cureus.70279




