Zeatin Analysis Service
Creative Proteomics offers high-precision zeatin analysis services using advanced LC-MS/MS (Sciex QTRAP 6500+) and UHPLC (Agilent 1290 Infinity II) platforms, enabling absolute quantification of over 20 cytokinin-related metabolites across diverse plant matrices. Our targeted cytokinin profiling helps plant scientists, breeders, and crop engineers uncover hormone regulation mechanisms, evaluate stress responses, and accelerate trait selection through accurate, reproducible, and customizable metabolic data.
Submit Your Request Now
×- What We Provide
- Advantage
- Workflow
- Technology Platform
- Sample Requirements
- Demo
- FAQ
- Case Study
- Publications
What is Zeatin?
Zeatin is a naturally occurring cytokinin, a class of plant hormones pivotal in regulating cell division, shoot initiation, leaf senescence, and other key physiological processes. As a derivative of adenine, zeatin plays a critical role in modulating gene expression in plant development, stress response, and yield optimization. Zeatin exists in several isoforms, including trans-zeatin (tZ), cis-zeatin (cZ), and their corresponding riboside and nucleotide derivatives, each exhibiting unique biological activities.
Why Analyze Zeatin?
Accurate quantification of zeatin and its derivatives is essential for plant scientists, agronomists, and agricultural biotechnology developers seeking to:
- Optimize Plant Growth and Productivity: Understand hormonal balances that influence morphogenesis.
- Elucidate Stress Response Mechanisms: Study adaptive responses to biotic and abiotic stresses.
- Accelerate Breeding Programs: Profile cytokinins in genetically modified and hybrid lines.
- Enhance Crop Engineering: Correlate endogenous cytokinin levels with transgenic expression targets.
Zeatin Analysis Service Offered by Creative Proteomics
Targeted Quantification of Zeatin and Its Derivatives
- Absolute quantification of major zeatin forms, including trans-zeatin (tZ), cis-zeatin (cZ), and their riboside, monophosphate, and glucoside conjugates.
- High-throughput detection using internal standard calibration.
Multi-Matrix Zeatin Profiling
- Tailored extraction and detection from various plant matrices such as roots, leaves, stems, seeds, fruits, sap, and culture media.
- Matrix-specific method optimization to ensure data reliability.
Zeatin-Related Cytokinin Panel Analysis
- Simultaneous measurement of over 20 cytokinin-related metabolites (e.g., iP, iPR, tZMP, cZR).
- Useful for mapping hormone biosynthesis and interconversion.
Cytokinin Metabolic Pathway Mapping
- Integration of zeatin data into known biosynthetic and degradation pathways.
- Identification of key metabolic fluxes and bottlenecks.
Customized Hormone Profiling Panels
- Build your own cytokinin detection panel to include specific metabolites of interest.
- Option to combine with auxin, gibberellin, and ABA profiling in multiplex formats.
Stable Isotope Labeling for Dynamic Studies
- Quantitative analysis of zeatin turnover and biosynthesis kinetics using ¹³C- or ¹⁵N-labeled precursors.
- Applicable in metabolic flux analysis and hormone transport studies.
Batch Analysis and Comparative Studies
- High-throughput analysis for large-scale projects such as breeding trials, treatment screenings, and transgenic evaluations.
- Includes statistical reporting and PCA/PLS analysis upon request.
Quality-Controlled Data Reporting
- Delivery of raw and processed data, calibration curves, QC metrics, and customizable result formats.
- Optional pathway annotation and graphical visualization (heatmaps, volcano plots, etc.).
List of Detected Zeatin and Related Metabolites
| Category | Compound Name | Biological Role / Pathway |
|---|---|---|
| Active Cytokinins | Trans-zeatin | Bioactive cytokinin; promotes cell division and growth |
| Cis-zeatin | Less active form; involved in specific tissue regulation | |
| Dihydrozeatin | Hydrogenated form; enhances shoot regeneration | |
| Kinetin | Synthetic analog; stimulates plant cell proliferation | |
| Riboside Derivatives | Trans-zeatin riboside | Transportable form of tZ in xylem |
| Cis-zeatin riboside | Mobile conjugate of cZ | |
| Dihydrozeatin riboside | Involved in cytokinin translocation | |
| Nucleotide Precursors | Trans-zeatin monophosphate | Intermediate in zeatin biosynthesis |
| Cis-zeatin monophosphate | Precursor in cZ biosynthetic pathway | |
| Isopentenyladenosine monophosphate | Biosynthetic precursor in IPT pathway | |
| Isopentenyl Cytokinins | Isopentenyladenine | Major precursor in cytokinin biosynthesis |
| Isopentenyladenosine | Transportable form of iP | |
| O-/N-Glucoside Conjugates | Trans-zeatin-O-glucoside | Inactive storage form |
| Cis-zeatin-O-glucoside | Reversible conjugate form | |
| Trans-zeatin-N-glucoside | Irreversible inactivation product | |
| Cis-zeatin-N-glucoside | Detoxification product | |
| Methylated/Degraded Products | Zeatin-7-glucoside | Minor metabolic product |
| Zeatin-9-glucoside | Breakdown product of zeatin | |
| Adenine derivatives | Structural core of all cytokinins | |
| Pathway-Associated Enzymes | IPT pathway intermediates | Biosynthesis via isopentenyl transferase |
| LOG pathway intermediates | Conversion of inactive forms to active bases | |
| CKX degradation products | Oxidative cleavage of cytokinins |
Advantages of Zeatin Assay
- Ultra-sensitive detection capability, with a lower limit of quantification as low as 0.1 pg/g fresh weight using advanced LC-MS/MS technology.
- Broad linear dynamic range, enabling accurate quantification from 0.1 pg/mL up to 10,000 pg/mL across all cytokinin isoforms.
- High precision and reproducibility, with intra-assay coefficient of variation (CV) consistently below 8%.
- Excellent calibration accuracy, supported by R² values exceeding 0.998 in all standard curves.
- Simultaneous detection of over 20 cytokinin-related metabolites, including active forms, precursors, conjugates, and degradation products.
- Validated for diverse plant matrices, covering more than 12 sample types such as leaf, root, seed, fruit, culture medium, and exudate.
- Absolute quantification enabled by internal stable isotope standards, including [²H]-labeled and [¹³C]-labeled zeatin analogs.
- Comprehensive data package, including raw signal files, quantitative result tables, QC metrics, and pathway-based visual summaries.
Workflow for Zeatin Analysis Service
Technology Platform for Zeatin Analysis Service
Primary Platform: LC-MS/MS using Sciex QTRAP 6500+ offers femtomole-level sensitivity and excellent reproducibility.
Chromatographic System: UHPLC system (Agilent 1290 Infinity II) equipped with C18 reversed-phase columns for sharp peak resolution.

SCIEX Triple Quad™ 6500+ (Figure from Sciex)

Agilent 1260 Infinity II HPLC (Fig from Agilent)
Sample Requirements for Zeatin Analysis Service
| Sample Type | Minimum Sample Amount | Notes |
|---|---|---|
| Fresh Plant Tissue (leaf, root, stem) | ≥ 500 mg fresh weight | Store at -80°C immediately after collection; avoid repeated freeze-thaw. |
| Freeze-Dried Plant Tissue | ≥ 100 mg dry weight | Grind into fine powder; ship with desiccant if possible. |
| Seeds | ≥ 100 mg | Homogenize if possible; pooled samples recommended for small seeds. |
| Fruits or Pods | ≥ 1 g fresh weight | Cut into smaller pieces; flash-freeze in liquid nitrogen. |
| Xylem or Phloem Sap | ≥ 500 µL | Collect in sterile, RNase-free tubes; store at -80°C. |
| Cell or Callus Culture | ≥ 1 × 10⁷ cells or 500 mg pellet | Centrifuge and wash with cold PBS; freeze immediately. |
| Culture Media / Exudates | ≥ 2 mL | Filtered supernatant; no antibiotics or additives. |
| Microdissected Plant Parts | As available; consult technical support | Ultra-micro analysis available; custom protocol required. |
Demo
Applications of Zeatin Assay Service
Agriculture
Optimize zeatin dosages in fertilizers to enhance crop yield and drought tolerance.
Plant Tissue Culture
Standardize zeatin concentrations for efficient callus induction and shoot regeneration.
Plant Physiology Studies
Elucidate zeatin's role in signaling pathways regulating cell division and senescence.
Plant Physiology Studies
Elucidate zeatin's role in signaling pathways regulating cell division and senescence.
Food Science
Monitor zeatin levels in post-harvest crops to extend shelf life and reduce spoilage.
Basic Research
Investigate zeatin metabolism (e.g., glycosylation) in model organisms like Arabidopsis.
FAQ of Zeatin Analysis Service
Can I submit mixed tissue types in the same project?
Yes. We accept projects with multiple tissue types (e.g., roots, leaves, seeds), but please submit each sample type separately and clearly labeled. Mixed tissues in a single tube are not recommended due to matrix effects.
What's the optimal time point for sampling in plant hormone studies?
Zeatin levels fluctuate diurnally and developmentally. We recommend sampling at consistent times of day and at defined growth stages. For time-course studies, replicate sampling across intervals (e.g., 0, 12, 24, 48 hours) is ideal.
How should I store and ship my samples?
Samples should be immediately frozen in liquid nitrogen and stored at –80°C. For shipment, use dry ice and ensure overnight delivery. Avoid repeated freeze-thaw cycles to preserve hormone integrity.
Can Creative Proteomics assist with experimental design?
Yes. Our scientists offer complimentary consultation to optimize sampling strategy, biological replication, and analytical scope based on your specific objectives.
Is absolute quantification available for all metabolites?
Absolute quantification is available for most key zeatin compounds (e.g., tZ, cZ, tZR, tZMP) via stable isotope-labeled internal standards. Some minor metabolites may be quantified relatively depending on standard availability.
How are results delivered and in what format?
You will receive a comprehensive digital report including raw data, quantification tables (Excel/CSV), signal traces, calibration curves, and optional pathway visualizations (PDF or image format).
Can I integrate Zeatin analysis with other phytohormone panels?
Yes. We offer multiplex hormone profiling services that can include auxins, gibberellins, abscisic acid, salicylic acid, and brassinosteroids. Please inquire for a custom panel.
Do you offer statistical or biological interpretation of the results?
We provide optional statistical services such as PCA, clustering, and pathway enrichment. Biological interpretation (e.g., regulation insights) can be discussed in follow-up consultation.
How many replicates should I submit?
We recommend a minimum of 3 biological replicates per condition for statistical robustness. Technical replicates are optional but not usually necessary due to our high instrument precision.
What if my sample volume is limited?
For rare or precious samples, we offer low-input analysis workflows. Please contact us in advance so we can assess feasibility and suggest minimal sample preparation protocols.
Can you re-analyze stored samples if I need additional data later?
Yes. We retain extracted sample aliquots (if volume permits) for up to 3 months after initial analysis. Re-analysis or expansion to new analytes is possible within this timeframe.
Do I need to provide reference standards or controls?
No, we supply all internal standards and calibration materials. However, you may provide your own controls if you wish to include custom references in the same analytical run.
Learn about other Q&A.
Zeatin Analysis Service Case Study

Title: A high trans-zeatin nucleoside concentration in corms may promote the multileaf growth of Amorphophallus muelleri.
Journal: Frontiers in Plant Science
Published: 2024
- Background
- Materials & Methods
- Results
Amorphophallus muelleri, a pearl-bud-type konjac, exhibits a unique multileaf growth pattern, unlike the single-leaf-per-season growth seen in most konjac species like A. konjac. This sequential leaf emergence enhances corm development and glucomannan yield, offering significant agronomic and economic benefits. However, the hormonal mechanisms governing this trait remain unclear. The study focuses on the dynamics of plant hormones—particularly cytokinins (notably trans-zeatin riboside, tZR), indole-3-acetic acid (IAA), and abscisic acid (ABA)—during different stages of leaf sprouting. It investigates how shifts in hormone concentrations and related gene expressions in corms correlate with axillary bud activation and multileaf development in A. muelleri.
Plant Growth and Sampling:
Two-year-old A. muelleri corms (~100 g) were cultivated in 10 L pots in a greenhouse (Kunming University, China) from May to October 2021 under 25–35°C, 50% shade, and 60–80% humidity. Developmental stages sampled included dormancy, sprouting, first and second leaf maturation, and lodging. Leaves and corm tissues were collected accordingly, with three biological replicates per stage.
Endogenous Phytohormone Analysis:
Phytohormones were extracted from frozen samples using acetonitrile-based solution with isotopically labeled standards. After homogenization and centrifugation, samples were filtered and analyzed using UHPLC-MS/MS (Waters ACQUITY UPLC with SCIEX systems). Calibration curves ensured quantification accuracy; precision was confirmed via RSD and recovery rates.
RNA-Seq Analysis:
RNA was extracted from corm tissues at four growth stages and sequenced using Illumina NovaSeq6000. Clean reads were aligned to the konjac reference genome (PRJNA608095) using HISAT2, and transcript abundance was quantified by FPKM via StringTie and RSEM. Differential expression was defined as adjusted P < 0.05 and fold change >2.
Exogenous iP Application:
Dormant corms (~5 g) were treated with 50 or 100 mg L⁻¹ iP for 3 h, then grown under controlled conditions (28°C, 16/8 h photoperiod). After 30 days, growth parameters (bud/root number and length) were measured. Hormone levels were analyzed in treated tissues.
Statistical Analysis:
One-way ANOVA and Tukey's test (P ≤ 0.05) were applied using SPSS 16.0. Heatmaps and plots were created with TBtools and SigmaPlot 10.0.
Cytokinin Dynamics:
Five cytokinins (KT, tZR, tZ, iP, iPA) showed stage-specific changes. In corms, KT, tZR, and tZ increased during early growth, with tZR peaking sharply (>5000× dormant level) at the second leaf sprouting stage. iP and iPA peaked in dormancy and decreased afterward. Leaf cytokinin levels were lower overall, with KT being the most abundant.
Effects of Exogenous iP:
Application of 100 mg L⁻¹ iP promoted bud/root growth and increased multibud formation. iP levels in buds were 30× higher than in corms under treatment, making up ~93% of total plant content. tZR also increased in buds. iP elevated IAA in corms and roots but reduced it in buds at low concentration. ABA decreased in buds and roots but rose in corms.
Gene Expression in Cytokinin Pathways:
IPT3/5 were upregulated in the second leaf mature stage. CYP735A1/2 showed strong induction during the second leaf sprouting stage. LOG expression rose moderately. CKX3/5/6 and UGT75L6, involved in cytokinin degradation, declined as the second leaf sprouted.
IAA and ABA Patterns:
IAA peaked in corms at the second leaf sprouting stage, correlating with high AO1 and PIN1A expression. ABA was highest in dormant corms and dropped sharply with bud emergence. NCED1 expression decreased, while ABA-degrading genes (CCD, PP2C51) increased during sprouting.
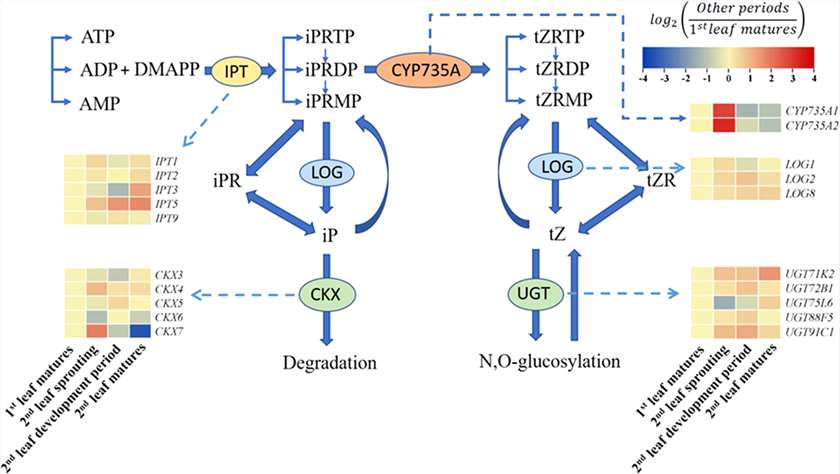 Summary of the cytokinin biosynthesis and catabolism pathways.
Summary of the cytokinin biosynthesis and catabolism pathways.
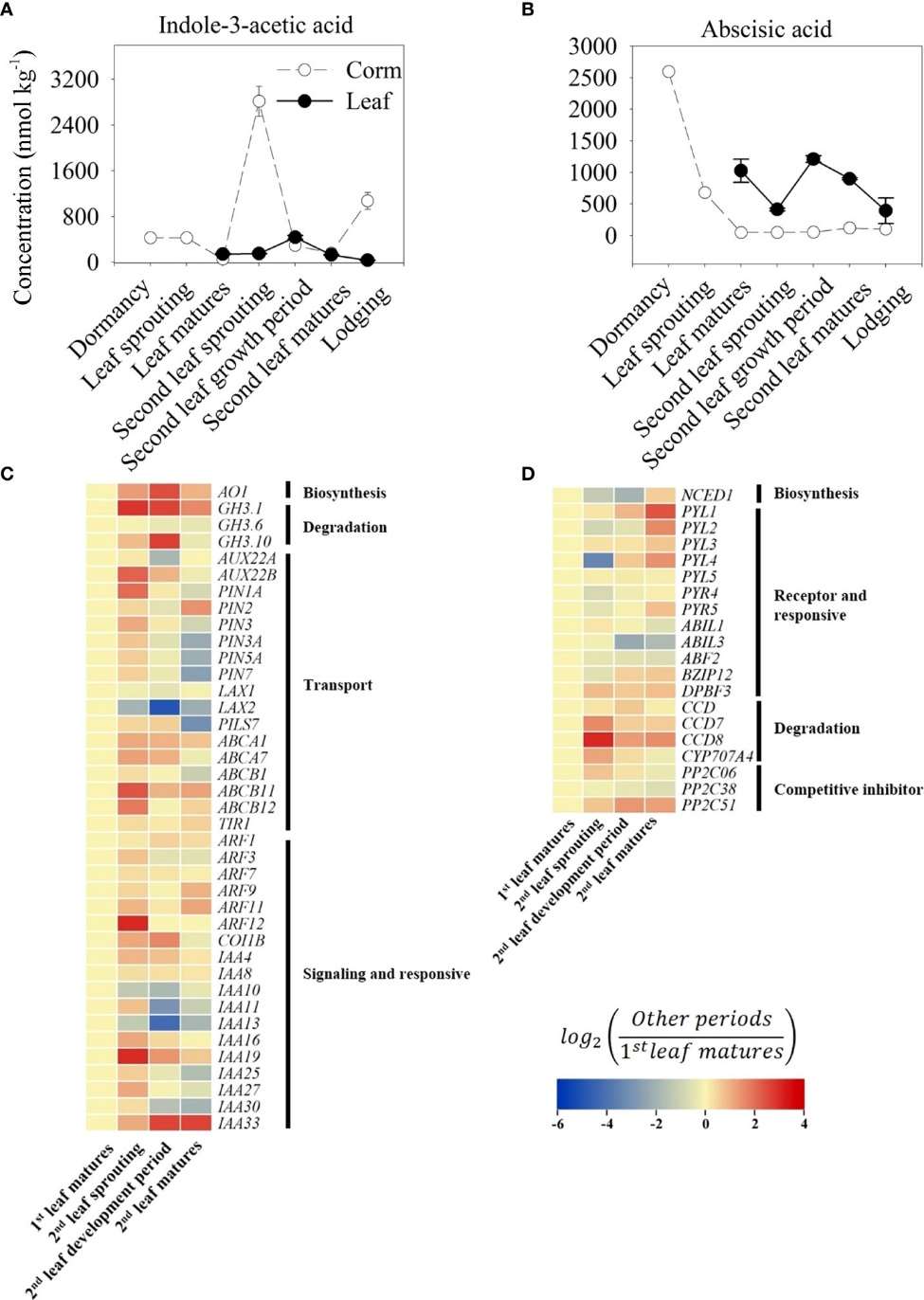 Trends of indole-3-acetic acid (IAA, A) and abscisic acid (ABA, B) content in growth cycle of A. muelleri.
Trends of indole-3-acetic acid (IAA, A) and abscisic acid (ABA, B) content in growth cycle of A. muelleri.
Reference
- Xue, Zhiqin, et al. "A high trans-zeatin nucleoside concentration in corms may promote the multileaf growth of Amorphophallus muelleri." Frontiers in Plant Science 13 (2022): 964003. https://doi.org/10.3389/fpls.2022.964003
Publications
Here are some publications in Metabolomics research from our clients:

- Choleoeimeria pogonae Alters the Bile Acid Composition of the Central Bearded Dragon (Pogona vitticeps). 2021. https://doi.org/10.5818/JHMS-D-20-00009
- Water-soluble saponins accumulate in drought-stressed switchgrass and may inhibit yeast growth during bioethanol production. 2022. https://doi.org/10.1186/s13068-022-02213-y
- Quantifying forms and functions of intestinal bile acid pools in mice. 2024. https://doi.org/10.1101/2024.02.16.580658
- Non-invasive elevation of circulating corticosterone increases the rejection of foreign eggs in female American robins (Turdus migratorius). 2022. https://doi.org/10.1016/j.yhbeh.2022.105278
- Living in extreme environments: a photosynthetic and desiccation stress tolerance trade-off story, but not for everyone. 2023. https://doi.org/10.22541/au.168311184.42382633/v2








