Xylose Analysis Service
Creative Proteomics delivers precise, pathway-integrated analysis of xylose and related metabolites—empowering biomass conversion, microbial engineering, and fermentation process control.
We provide:
- Accurate quantification of D-xylose, xylitol, and sugar phosphates
- Pathway-specific insights into carbon flux and metabolite turnover
- Broad sample compatibility across plant, microbial, and fermentation systems
We solve:
- Inaccurate xylose quantification in complex matrices
- Bottlenecks in microbial xylose utilization
- Unresolved pathway dynamics in engineered systems
Submit Your Request Now
×
What You Will Receive
- Raw and processed data (peak tables, QC metrics)
- Absolute/relative quantification reports
- Annotated pathway enrichment maps
- Multivariate statistics (PCA, volcano plot)
- Time-course curves and flux trend visualizations
- What We Provide
- Advantages
- Technology Platform
- Sample Requirements
- Demo
- FAQs
- Case Study
What Is Xylose?
Xylose is a five-carbon monosaccharide and a key building block of plant hemicellulose. As one of the most abundant sugars in lignocellulosic biomass, it plays a central role in plant structural integrity and carbon cycling. In microbial systems, xylose serves as a major alternative carbon source—especially in engineered strains for bio-based production.
However, xylose and its metabolic intermediates are chemically similar to other pentoses and sugar alcohols, making accurate quantification in complex matrices technically challenging. Advanced analytical methods are essential for resolving its metabolic roles across industrial, agricultural, and environmental contexts.
Why Perform Xylose Analysis?
Precise measurement of xylose and its derivatives supports a wide range of scientific and applied objectives:
- Strain optimization: Quantify xylose uptake and catabolism in engineered microbes or yeast strains for improved fermentation output.
- Plant biomass characterization: Evaluate xylose content in crop residues and energy crops to guide biomass pretreatment and enzymatic hydrolysis strategies.
- Microbial metabolism studies: Monitor xylose-driven metabolic flux and its impact on short-chain fatty acid (SCFA) production in microbial communities.
- Pathway reconstruction: Integrate xylose and its phosphorylated intermediates (e.g., xylulose-5-phosphate) into pentose phosphate pathway models for systems biology and metabolomics research.
- Bioprocess monitoring: Track xylose consumption and byproduct formation in fermentation workflows for biopolymer, xylitol, or ethanol production.
Creative Proteomics offers targeted, high-resolution xylose metabolomics to help researchers and process developers generate meaningful, pathway-informed insights from plant, microbial, and environmental samples.
Xylose Analysis Service Offered by Creative Proteomics
Quantitative Analysis of D-Xylose and Related Metabolites
Accurate quantification of D-xylose, xylulose, xylitol, and other downstream metabolites in complex biological matrices such as plasma, urine, plant extracts, fermentation broth, and microbial cultures.
Multiplex Profiling of Xylose Metabolic Intermediates
Targeted analysis of critical intermediates in xylose metabolism pathways, including:
- Xylulose-5-phosphate
- Ribulose-5-phosphate
- Sedoheptulose-7-phosphate
These intermediates are essential in the pentose phosphate pathway and carbon flux analysis.
Detection of Fermentation Byproducts and Terminal Compounds
Quantification of short-chain fatty acids (e.g., acetate, lactate, butyrate) and end-products like glycerol and pyruvate generated during microbial xylose fermentation or metabolic engineering experiments.
Microbial Xylose Utilization Profiling
Designed for microbial screening and strain optimization, this service evaluates xylose uptake, catabolism, and pathway efficiency. Stable isotope tracing (e.g., ¹³C-xylose) is available upon request to support metabolic flux analysis.
Pathway Enrichment and Regulatory Network Analysis
Integration of metabolite data with pathway databases to support mechanistic interpretation of xylose-related regulatory networks—useful in gut microbiota studies, plant stress research, and intestinal absorption assessments.
Custom Marker Panel Development
Development of tailored xylose biomarker panels to support specific research goals, such as oral xylose tolerance testing (XTT), nutritional absorption studies, or engineered cell line validation.
List of Detected Xylose and Related Metabolites
| Category | Representative Metabolites | Associated Pathways |
|---|---|---|
| Monosaccharides | D-Xylose, L-Arabinose, D-Ribose, D-Glucose, D-Galactose | Pentose metabolism, Glycolysis |
| Sugar Alcohols | Xylitol, Arabinitol, Ribitol, Sorbitol | Polyol pathway, Osmoregulation |
| Sugar Acids | D-Xylonic acid, L-Arabinonic acid, D-Gluconic acid | Oxidative carbohydrate metabolism |
| Phosphate Esters | Xylulose-5-phosphate, Ribulose-5-phosphate, Sedoheptulose-7-phosphate, Glyceraldehyde-3-phosphate | Pentose phosphate pathway, Calvin cycle |
| Fermentation Byproducts | Acetate, Lactate, Formate, Ethanol, Butyrate | Anaerobic fermentation, SCFA metabolism |
| Central Carbon Intermediates | Pyruvate, Phosphoenolpyruvate, Dihydroxyacetone phosphate (DHAP), Citrate, Malate | Glycolysis, TCA cycle, Gluconeogenesis |
| Nucleotide Sugars | UDP-Xylose, UDP-Glucose, UDP-Galactose | Glycosylation, Cell wall biosynthesis |
| Methylated Sugars / Derivatives | 2-Methyl-D-erythritol, 3-Deoxy-D-manno-octulosonate (KDO) | Microbial cell wall biosynthesis |
| Lignocellulose-Derived Markers | Furfural, Hydroxymethylfurfural (HMF), Levulinic acid | Biomass degradation, Pretreatment byproducts |
| Stable Isotope Tracers (optional) | ¹³C5-D-Xylose, ¹³C3-Xylitol, ¹³C1-Acetate | Fluxomics, Metabolic labeling studies |
Advantages of Xylose Assay
- Low Detection Limit: Detection of D-xylose down to<1.0 ng/mL in plasma, urine, and fermentation media using LC-MS/MS.
- Broad Quantification Range: Dynamic range spans 5–6 orders of magnitude, allowing for simultaneous detection of trace metabolites and highly abundant intermediates.
- High Sample Compatibility: Validated across 10+ sample types, including serum, feces, urine, microbial cultures, fermentation broth, and pretreated lignocellulose hydrolysates.
- Comprehensive Pathway Coverage: Quantifies >60 metabolites directly or indirectly involved in xylose metabolism, spanning the pentose phosphate pathway, glycolysis, TCA cycle, and short-chain fatty acid biosynthesis.
- High Reproducibility: Intra-batch CV (coefficient of variation) typically<10% for most quantified compounds under optimized conditions.
- Optional Stable Isotope Tracing: Supports ¹³C-labeled xylose tracing for advanced fluxomics and metabolic engineering studies.
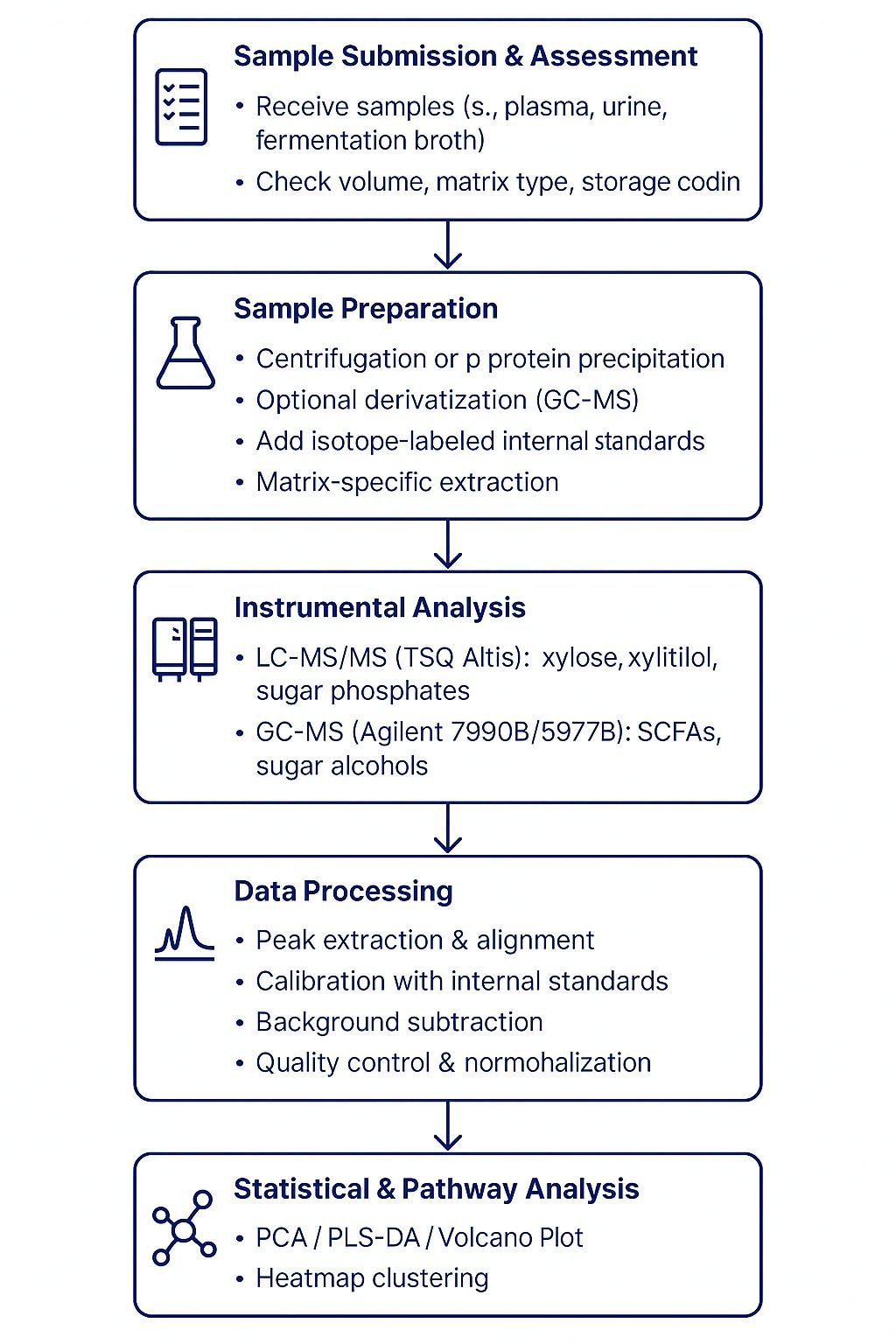
Workflow for Xylose Analysis Service
Technology Platform for Xylose Analysis Service
LC-MS/MS (Liquid Chromatography–Tandem Mass Spectrometry)
Instrument Model: Thermo Scientific TSQ Altis Triple Quadrupole MS
Application: Targeted quantification of xylose, xylitol, xylulose-5-phosphate, and related sugar phosphates in biological fluids and microbial extracts.
Advantages:
- High specificity via multiple reaction monitoring (MRM)
- Low detection limit (LOD < 1 ng/mL)
- Suitable for polar, non-volatile metabolites
 TSQ Altis Triple Quadrupole MS (Figure from Thermo Scientific)
TSQ Altis Triple Quadrupole MS (Figure from Thermo Scientific)
GC-MS (Gas Chromatography–Mass Spectrometry)
Instrument Model: Agilent 7890B GC system with 5977B MSD
Application: Analysis of derivatized xylose, sugar alcohols, and fermentation byproducts such as acetate, butyrate, and furfural.
Advantages:
- Excellent resolution for volatile and semi-volatile compounds
- Ideal for fermentation monitoring and lignocellulose hydrolysate profiling
 Agilent 7890B-5977B (Figure from Agilent)
Agilent 7890B-5977B (Figure from Agilent)
Sample Requirements for Xylose Analysis Service
| Sample Type | Recommended Volume / Weight | Container | Storage Conditions | Notes |
|---|---|---|---|---|
| Plasma / Serum | ≥ 100 µL | 1.5 mL microcentrifuge tube | –80 °C | Use EDTA or heparin; avoid repeated freeze–thaw cycles |
| Urine | ≥ 200 µL | 1.5 mL microcentrifuge tube | –80 °C | Collect midstream; no preservatives |
| Feces | ≥ 100 mg | Sterile cryovial | –80 °C | Avoid dietary fiber contamination |
| Fermentation Broth | ≥ 1.0 mL | 2.0 mL tube or glass vial | –20 °C or lower | Filter or centrifuge before freezing |
| Microbial Cultures | ≥ 10⁸ cells (wet pellet) | 1.5 mL microcentrifuge tube | –80 °C | Wash with PBS to remove medium |
| Plant Tissue / Biomass | ≥ 100 mg (fresh or frozen) | Aluminum foil or cryovial | Liquid nitrogen or –80 °C | Rinse and blot before freezing |
| Food / Feed Samples | ≥ 200 mg (homogenized) | Sealed tube or vial | –20 °C | Remove moisture; avoid spoilage |
Demo Results
FAQ of Xylose Analysis Service
Can I submit multiple sample types in one project?
Yes. We support mixed sample batches (e.g., plasma + fermentation broth) as long as each sample type meets the respective handling and volume requirements. Please label and package each type separately.
Do I need to derivatize xylose samples before sending them?
No. Our lab handles all necessary derivatization steps in-house, tailored to the selected platform (e.g., GC-MS or LC-MS/MS). We recommend sending native, unprocessed samples.
Can you detect both xylose and its fermentation byproducts in the same run?
Yes. Our method panels can simultaneously capture D-xylose, xylitol, SCFAs (e.g., acetate, butyrate), and other downstream intermediates, depending on the chosen analytical platform.
How do I ensure sample stability during shipping?
We recommend using dry ice for biological samples and including internal cold packs for short-distance transport. For plant or microbial samples, flash-freezing in liquid nitrogen prior to shipping is preferred.
Is this service suitable for monitoring xylose metabolism in engineered microbes?
Absolutely. Our platform is frequently used in synthetic biology and metabolic engineering studies to track xylose utilization efficiency and redirect metabolic flux toward desired endpoints.
Can you accommodate high-throughput time-course studies?
Yes. We can process up to 96 samples per batch. Time-series data (e.g., fermentation time points or dietary intervention) can be analyzed with integrated normalization and statistical comparison.
What if my samples have very low xylose concentrations?
Our LC-MS/MS method has an LOD of<1 ng/mL, making it suitable for trace-level detection in clinical and environmental samples. We can recommend pre-concentration steps if needed.
Do you provide pathway-level interpretation along with the results?
Yes. Our standard deliverables include annotated pathway maps and enrichment analysis, helping you visualize how xylose-related metabolites integrate into broader metabolic networks.
Learn about other Q&A.
Xylose Analysis Service Case Study

Title: Insect derived extra oral GH32 plays a role in susceptibility of wheat to Hessian fly
Journal: Scientific Reports
Published: 2021
- Background
- Methods
- Results
- Reference
The Hessian fly (Mayetiola destructor) is a major obligate pest of wheat, causing significant global crop losses. Unlike chewing insects, Hessian fly larvae lack developed mandibles and instead rely on molecular mechanisms to convert host plant tissue into a nutrient-rich environment. However, the specific virulence factors that promote susceptibility in host wheat remain poorly characterized.
This study investigates the role of a horizontally transferred glycoside hydrolase gene, MdesGH32, expressed by virulent Hessian fly larvae. GH32 enzymes are known to exhibit invertase and inulinase activities that break down plant cell wall polysaccharides and convert transport sugars into monosaccharides.
This study combined entomological, molecular, and metabolomic techniques to investigate the mechanism of wheat susceptibility to Hessian fly (Mayetiola destructor) infestation.
Plant and Insect Materials
Four wheat lines (two resistant: Iris, Hamlet; two susceptible: Newton, Centurk) and non-host Brachypodium distachyon were used. Biotype L Hessian fly larvae were reared and infested under controlled conditions. Tissue samples were collected at multiple time points post-infestation for molecular and biochemical assays.
Gene Expression and Enzyme Profiling
- RNA was extracted from larvae and plant tissues for qRT-PCR analysis of GH32, GLUT (glucose transporter), TaFRK (fructokinase), and 29 cell wall biosynthesis-related genes.
- Enzyme activities (invertase, inulinase, levanase) were quantified from total larval protein and recombinant MdesGH32 protein using standard colorimetric assays.
Metabolomic Analysis by Creative Proteomics
Creative Proteomics provided the quantitative metabolomic profiling of plant sugars in infested and uninfested wheat samples:
- Analytes: D-glucose and D-fructose
- Sample matrix: Crown tissue from resistant (Hamlet) and susceptible (Centurk) wheat
- Sample preparation: 75% methanol extraction, steel-bead homogenization, sonication, centrifugation
- Quantification method:
- UPLC–(–)ESI–MRM/MS, using Agilent 1290 UHPLC coupled with Agilent 6495 QQQ mass spectrometer
- Internal standards: 6 U-¹³C-labeled analogs for glucose and fructose
- Calibration curves constructed from known standards for absolute quantification
- Findings:
- Glucose levels significantly increased (2.2-fold at 5 DAH, 1.6-fold at 8 DAH) in susceptible wheat after infestation.
- Fructose levels remained unchanged.
GH32 Expression and Secretion:
MdesGH32 was highly upregulated in virulent larvae feeding on susceptible wheat, with transcript levels peaking at 421-fold increase by 3 days after hatch (DAH). The protein was secreted extra-orally into the plant feeding site and localized within mesophyll and epidermal tissues.
Enzymatic Activity:
Recombinant MdesGH32 demonstrated strong invertase and inulinase activity, effectively converting sucrose into glucose and fructose. Enzymatic activity was significantly higher in virulent larvae compared to avirulent ones.
Metabolomic Evidence – UPLC-MS Analysis:
Using UPLC–(–)ESI–MRM/MS (Agilent 1290 UHPLC + 6495 QQQ), the authors observed a 2.2-fold increase in glucose in susceptible wheat by 5 DAH and 1.6-fold increase by 8 DAH, whereas fructose levels remained unchanged. Resistant plants did not show glucose elevation.
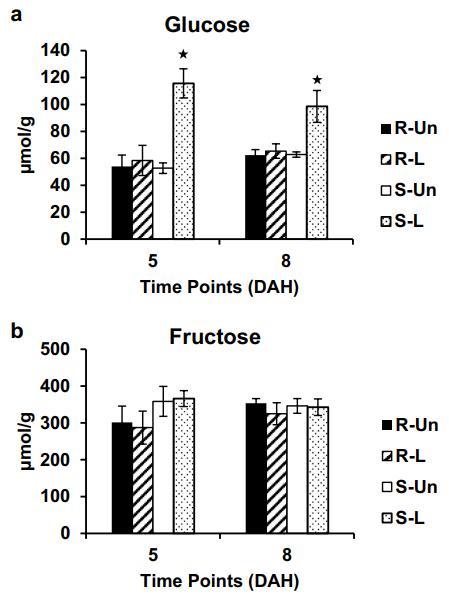 Wheat monosaccharide levels. (a) Glucose levels. (b) Fructose levels.
Wheat monosaccharide levels. (a) Glucose levels. (b) Fructose levels.
Physiological and Molecular Impact:
- Increased expression of TaFRK (fructokinase gene) in susceptible wheat confirmed metabolic channeling of fructose into glycolysis.
- Hessian fly glucose transporter gene (MdesGLUT) was also highly expressed, supporting enhanced glucose uptake by larvae.
- Neutral Red staining revealed elevated cell wall permeability in susceptible plants.
- Expression of cell wall biosynthetic genes was suppressed in susceptible wheat, indicating compromised structural integrity.
Reference
- Subramanyam, Subhashree, et al. "Insect derived extra oral GH32 plays a role in susceptibility of wheat to Hessian fly." Scientific Reports 11.1 (2021): 2081. https://doi.org/10.1038/s41598-021-81481-4
Publications
Here are some of the metabolomics-related papers published by our clients:

- Insect derived extra oral GH32 plays a role in susceptibility of wheat to Hessian fly. 2021. https://doi.org/10.1038/s41598-021-81481-4
- Modulation of the Endomembrane System by the Anticancer Natural Product Superstolide/ZJ-101. 2023. https://doi.org/10.3390/ijms24119575
- Identification of the O-Glycan Epitope Targeted by the Anti-Human Carcinoma Monoclonal Antibody (mAb) NEO-201. 2022. https://doi.org/10.3390/cancers14204999
- Alternative glycosylation controls endoplasmic reticulum dynamics and tubular extension in mammalian cells. 2021. https://doi.org/10.1126/sciadv.abe8349
- Conformational differences between functional human immunodeficiency virus envelope glycoprotein trimers and stabilized soluble trimers. 2019. https://doi.org/10.1128/jvi.01709-18











