Tanshinone Analysis Service
Creative Proteomics offers expert analytical services for tanshinones, including precise quantification, metabolite identification, and metabolic pathway analysis. We support the pharmaceutical, nutraceutical, and cosmetic industries by ensuring product quality, optimizing pharmacokinetics, and advancing drug development. Our advanced LC-MS/MS techniques help address challenges in consistency, efficacy, and safety of herbal extracts and formulations.
Submit Your Request Now
×- What We Provide
- Advantage
- Workflow
- Technology Platform
- Sample Requirements
- FAQ
- Case Study
What is Tanshinone?
Tanshinones are a class of lipophilic diterpenoid quinones predominantly isolated from Salvia miltiorrhiza (Danshen), a traditional Chinese herb with extensive pharmacological applications. Key bioactive constituents include tanshinone I (TANI), tanshinone IIA (TIIA), cryptotanshinone (CTAN), and dihydrotanshinone (DTAN). These compounds exhibit diverse biological activities, such as anti-inflammatory, antioxidant, and osteoprotective effects, making them critical targets for pharmaceutical and nutraceutical research.
Why Analyze Tanshinone?
Accurate quantification and metabolic profiling of tanshinones are essential for:
- Quality control of herbal extracts to ensure batch consistency.
- Mechanistic studies to elucidate their roles in signaling pathways (e.g., AKT1/PI3K modulation in osteoporosis).
- Metabolic stability assessment to predict pharmacokinetic behavior in preclinical models.
- Product development for nutraceuticals, cosmetics, and agrochemicals.
Tanshinone Analysis Service Offered by Creative Proteomics
- Quantification of Tanshinone Compounds: Accurate measurement of various tanshinone compounds in plant extracts, biological fluids, and pharmaceutical formulations.
- Identification of Tanshinone Metabolites: Comprehensive identification of tanshinone metabolites in biological matrices, facilitating a deeper understanding of their pharmacokinetics.
- Metabolomic Profiling: Detailed profiling of metabolites involved in tanshinone metabolism using advanced mass spectrometry and chromatography techniques.
- Tanshinone Pathway Analysis: Identification and analysis of the metabolic pathways associated with tanshinone, providing insights into its mechanism of action and bioavailability.
- In vitro Pharmacological Activity Evaluation: Assessment of the biological activity of tanshinone and its metabolites through in vitro assays, such as anti-inflammatory, antioxidant, and cytotoxicity testing.
- Standardization of Tanshinone Extracts: Standardization of tanshinone extracts to ensure consistency and quality in herbal products or pharmaceutical formulations.
List of Detected Tanshinone and Related Metabolites
| Compound Category | Detected Molecule | Metabolite(s) | Associated Pathways |
|---|---|---|---|
| Primary Tanshinones | Tanshinone I | Hydroxytanshinone I | Phase I oxidation (CYP450 enzymes) |
| Tanshinone IIA | Dihydro-Tanshinone IIA, Glucuronides | CYP-mediated oxidation, glucuronidation | |
| Tanshinone IIB | Hydroxy-Tanshinone IIB | Phase I hydroxylation | |
| Cryptotanshinone | 15-Hydroxycryptotanshinone | CYP3A4-mediated hydroxylation | |
| 15,16-Dihydrotanshinone I | Hydroxy-Dihydrotanshinone | Reductive metabolism, hydroxylation | |
| Secondary Metabolites | Miltirone | Demethylated Miltirone | Phase I demethylation |
| Neo-Tanshinlactone | Hydroxy-Neo-Tanshinlactone | Lactone ring oxidation | |
| Conjugated Metabolites | Tanshinone-glucuronide | — | UGT-mediated glucuronidation |
| Tanshinone-sulfate | — | Sulfotransferase-mediated conjugation | |
| Other Related Terpenoids | Dihydrofuran-Tanshinone Derivatives | Hydroxylated Tanshinone Derivatives | Mixed CYP pathways, aldehyde oxidation |
Advantages of Tanshinone Assay
- Ultra-Low Detection Limits: Detection sensitivity down to 0.1 ng/mL for major tanshinones using triple quadrupole LC-MS/MS systems (e.g., Agilent 6495C, Sciex QTRAP 6500+), enabling trace-level quantification in complex biological matrices.
- High Accuracy and Reproducibility: Intra-assay and inter-assay variability controlled within <5% RSD, supported by internal standards and isotopic calibration.
- Expanded Analyte Coverage: Capability to simultaneously quantify >20 tanshinone-related compounds and metabolites in a single run via optimized MRM panels.
- Wide Dynamic Range: Quantification range spans 0.1 ng/mL to 10 µg/mL, suitable for both low-dose pharmacokinetic studies and high-concentration extracts.
- Matrix Flexibility: Validated for diverse sample types, including plasma, urine, tissues, plant extracts, and herbal formulations, with recovery rates exceeding 85% for most matrices.
- Advanced Pathway Resolution: Integrated with metabolomic software and pathway databases, enabling automated mapping of over 30 metabolic transformations associated with tanshinones, including CYP450 interactions and Phase II conjugation.
- Custom Data Reporting: Optional delivery of raw data, peak area tables, standard curves, and metabolic pathway annotations, fully customizable to client requirements.
Workflow for Tanshinone Analysis Service
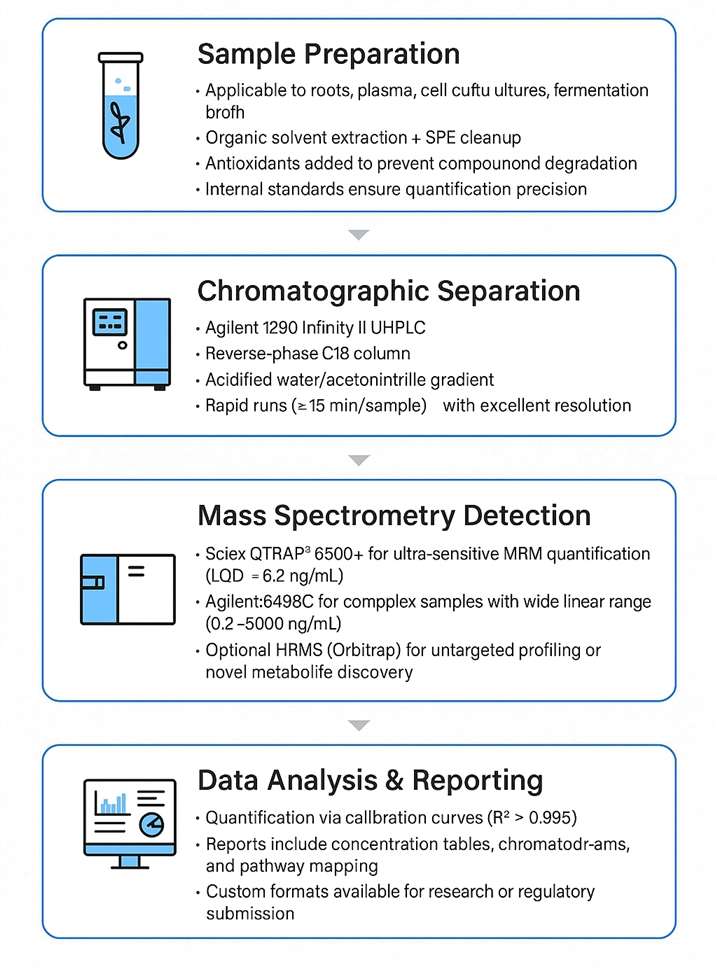
Technology Platform for Tanshinone Analysis Service
Sciex QTRAP® 6500+: Used for targeted quantification via LC-MS/MS with high sensitivity in MRM mode.
Agilent 6495C Triple Quadrupole LC-MS: Delivers precise quantification of low-abundance tanshinones in complex samples.
Agilent 1290 Infinity II: Supports high-throughput, high-resolution chromatography for tanshinone profiling.

SCIEX Triple Quad™ 6500+ (Figure from Sciex)

Agilent 6495C Triple Quadrupole (Figure from Agilent)

Agilent 1260 Infinity II HPLC (Fig from Agilent)
Sample Requirements for Tanshinone Analysis Service
| Sample Type | Minimum Volume / Weight | Storage Condition | Notes |
|---|---|---|---|
| Salvia miltiorrhiza root powder | ≥ 100 mg | Room temp (dry, sealed) | Preferably air-dried or lyophilized |
| Plant tissue (fresh/frozen) | ≥ 200 mg | –80°C | Rinse to remove soil/debris |
| Herbal extracts (liquid) | ≥ 500 µL | 4°C or –20°C | Include solvent composition |
| Blood plasma / serum | ≥ 200 µL | –80°C | Collected in EDTA tubes preferred |
| Urine | ≥ 1 mL | –80°C | Avoid repeated freeze-thaw |
| Fermentation broth / cell culture | ≥ 2 mL | –20°C or –80°C | Centrifuge to remove debris before shipping |
Applications of Tanshinone Assay Service
Herbal Medicine Research
Enables precise quantification of active tanshinones in Salvia miltiorrhiza for quality standardization and formulation development.
Natural Product Chemistry
Facilitates identification and characterization of novel tanshinone analogs and derivatives in complex plant extracts.
Metabolic Engineering
Supports pathway optimization and yield monitoring in tanshinone-producing microbial or plant-based expression systems.
Pharmacokinetics Studies
Measures in vivo distribution, bioavailability, and clearance of tanshinones in preclinical animal models.
Quality Control (QC)
Ensures batch-to-batch consistency and active ingredient verification in raw herbs, decoctions, and finished products.
Compound Stability Testing
Evaluates chemical stability of tanshinones under thermal, photolytic, oxidative, and acidic/basic stress conditions.
FAQ of Tanshinone Analysis Service
Can I test multiple tanshinones at once?
Yes. Our method quantifies several major tanshinones (e.g., I, IIA, cryptotanshinone) in a single LC-MS/MS run with high accuracy.
What types of samples can you analyze?
We support plant tissues, extracts, fermentation broth, plasma, urine, and even engineered cell cultures. Let us know your matrix in advance.
How much sample do I need to send?
We require:
- 100–200 mg for solid samples (e.g., powdered root)
- 200–500 µL for liquids (e.g., serum, extract)
We can advise if you have less than the recommended amount.
Do I need to know the tanshinone content beforehand?
No. We can perform qualitative screening first, then proceed to quantitative analysis based on your sample's composition.
Can I submit ethanol or methanol-preserved samples?
Yes. Alcohol-based preservation is acceptable and helps stabilize tanshinones. Please label solvent type and concentration clearly.
Will I receive both raw and processed data?
Yes. Deliverables include full quantitative reports (PDF + Excel), plus raw LC-MS data files upon request (.mzML, .raw, or native format).
How do I compare different batches or treatments?
We offer batch comparison reports with normalized concentrations, bar charts, and variance analysis across your sample groups.
How are samples shipped and stored?
Ship dry samples at room temperature; biological materials should be shipped frozen on dry ice. We offer short-term storage (≤30 days) for leftover samples.
Can I run a pilot test first?
Yes. Pilot testing is encouraged for new sample types or unknown matrices. It ensures method compatibility and saves time in large-scale projects.
Learn about other Q&A.
Tanshinone Analysis Service Case Study

Title: Investigation on the Metabolism of Salvianolic Acid B, Tanshinone ⅡA, Ginsenoside Rg1, and Ginsenoside Rb1 from Danshen-Sanqi Herbal Pair in Zebrafish by Liquid Chromatography-Tandem Mass Spectrometry Analysis
Journal: Clinical Complementary Medicine and Pharmacology
Published: 2021
- Background
- Materials & Methods
- Results
- Conclusion
The study focuses on the active components of the Danshen-Sanqi herbal pair (DS-SQ), namely Salvianolic acid B (SAB), Tanshinone IIA (TIIA), Ginsenoside Rg1 (GRg1), and Ginsenoside Rb1 (GRb1). These compounds are used for treating cardiovascular diseases in Asia. The research aims to investigate the metabolism of these compounds in zebrafish, a model organism known for its small size, fast reproduction, and transparent embryos. Zebrafish are increasingly used in drug metabolism research due to their similarity to human genetic sequences and the ability to express drug-metabolizing enzymes. By utilizing liquid chromatography-tandem mass spectrometry (LC-MS/MS), the study explores the metabolic pathways of SAB, TIIA, GRg1, and GRb1 and investigates their potential synergistic effects when combined.
Chemicals and Reagents
Ginsenoside Rg1, Rb1, SAB, and TIIA were sourced from respective suppliers. DMSO, acetonitrile, and formic acid (HPLC grade) were from Chengdu Chron Chemicals and Beijing InnoChem. Water was purified.
Metabolite analysis was performed using a Shimadzu 8060 Triple-Quadruple mass spectrometer with ESI interface. Separation was achieved with an Agilent Zorbax SB-Aq column. The mass spectra were recorded in both positive and negative ionization modes (m/z 100-2000) with 50% collision energy.
Drug Administration and Biological Sample Preparation
Zebrafish larvae (48 hours post-hatching) were exposed to 8 µM drug solutions. After homogenization and centrifugation, the supernatant was treated with methanol to remove proteins and filtered before LC-MS/MS analysis.
Statistical Analysis
Data are expressed as mean ± SD. Statistical analysis was performed using SPSS.
Eighteen metabolites and four parent compounds of SAB, TIIA, GRg1, and GRb1 were identified in zebrafish using HPLC-MS/MS. Seven SAB metabolites, including SAB-1 (monomethyl-SAB), SAB-2 (tanshinol), and SAB-5 (lithospermic acid), were detected. TIIA metabolites included TIIA-1 (hydroxylated TIIA) and TIIA-2 (glucuronide conjugate). GRg1 metabolites, such as GRg1-1 (Rh1/F1) and GRg1-2 (Protopanaxatriol), and GRb1 metabolites (e.g., GRb1-2, GRb1-3) were identified. Metabolic interactions between the drugs revealed that GRg1 and GRb1 decreased certain SAB metabolites while increasing others. TIIA and GRg1/GRb1 co-treatment altered the levels of TIIA and GRg1 metabolites.
Table 1 Identification of the metabolites of SAB, TIIA, GRg1 and GRb1 in zebrafish by HPLC-MS/MS
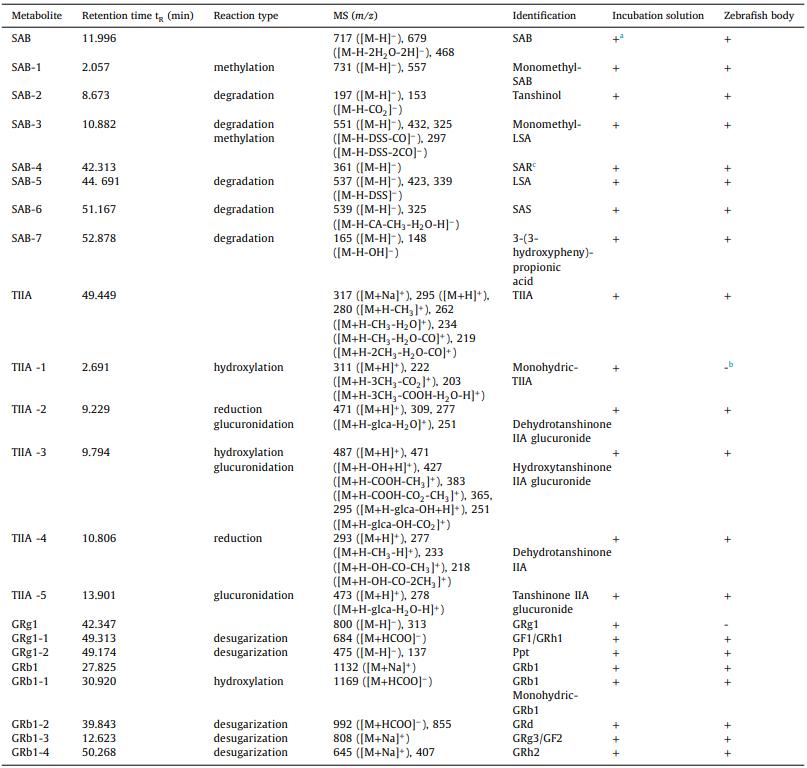
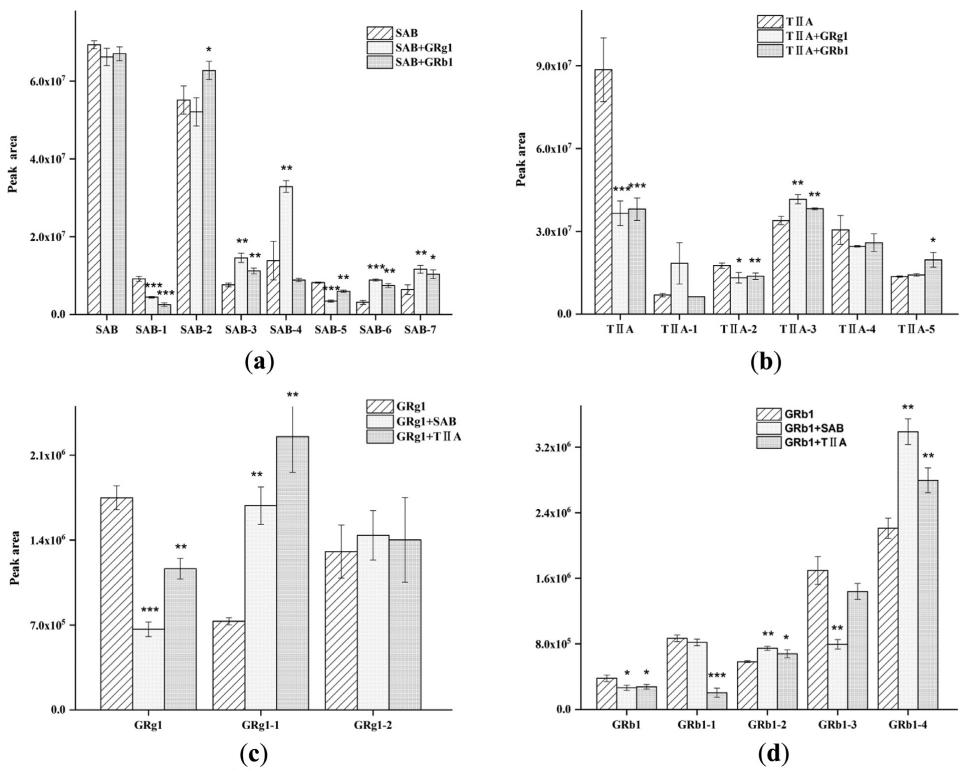 Fig. Peak areas of SAB (a), TIIA (b), GRg1 (c), and GRb1 (d) metabolites after single and combined exposure in zebrafish (n=3).
Fig. Peak areas of SAB (a), TIIA (b), GRg1 (c), and GRb1 (d) metabolites after single and combined exposure in zebrafish (n=3).
Metabolic interactions between SAB, TIIA, GRg1, and GRb1 suggest changes in metabolic pathways that may enhance therapeutic effects, particularly in cardiovascular disease. The increased metabolite levels could offer potential adjunctive treatments for human diseases, including cancer and cardiovascular conditions, due to their favorable safety and efficacy profiles.
Reference
- Yin, Shijun, et al. "Investigation on the Metabolism of Salvianolic acid B, Tanshinone ⅡA, Ginsenoside Rg1 and Ginsenoside Rb1 from Danshen-Sanqi Herbal Pair in Zebrafish by Liquid Chromatography-Tandem Mass Spectrometry Analysis." Clinical Complementary Medicine and Pharmacology 1.1 (2021): 100001. https://doi.org/10.1016/j.ccmp.2021.100001
Publications
Here are some publications in Metabolomics research from our clients:

- Elevated SLC7A2 expression is associated with an abnormal neuroinflammatory response and nitrosative stress in Huntington’s disease. 2024. https://doi.org/10.1186/s12974-024-03038-2
- Pan-lysyl oxidase inhibition disrupts fibroinflammatory tumor stroma, rendering cholangiocarcinoma susceptible to chemotherapy. 2024. https://doi.org/10.1097/HC9.0000000000000502
- Exogenous lipase administration alters gut microbiota composition and ameliorates Alzheimer’s disease-like pathology in APP/PS1 mice. 2022. https://doi.org/10.1038/s41598-022-08840-7
- Glucocorticoid-induced osteoporosis is prevented by dietary prune in female mice. 2024. https://doi.org/10.3389/fcell.2023.1324649
- Polyamine metabolism impacts T cell dysfunction in the oral mucosa of people living with HIV. 2023. https://doi.org/10.1038/s41467-023-36163-2







