Cryptotanshinone Analysis Service
Creative Proteomics provides high-precision LC-MS/MS analysis services for plant extracts and natural products. We offer Cryptotanshinone quantification, metabolic pathway mapping, and quality control, helping clients address issues of accuracy, stability, and batch consistency in plant ingredient identification, standardization, synthetic biology, and pharmacological research. Our services deliver fast, reliable data to support product quality and efficient research progress.
Submit Your Request Now
×- What We Provide
- Advantage
- Workflow
- Technology Platform
- Sample Requirements
- FAQs
- Case Study
What is Cryptotanshinone?
Cryptotanshinone (CT) is a bioactive diterpenoid quinone extracted predominantly from Salvia miltiorrhiza (Danshen), a traditional Chinese medicinal herb. Structurally characterized by its phenanthraquinone core, Cryptotanshinone has been of great interest due to its diverse biological activities, including modulation of oxidative stress, cellular signal transduction, and enzyme inhibition. Its lipophilic nature and complex biosynthetic background necessitate high-precision analytical techniques to ensure accurate characterization, quantification, and quality control.
Why Analyze Cryptotanshinone?
Understanding and quantifying cryptotanshinone is vital in natural product research, botanical extract standardization, metabolic engineering, and pharmacognosy. Rigorous analytical services are necessary to:
- Authenticate raw materials and botanical formulations.
- Monitor bioconversion efficiency in synthetic biology or fermentation processes.
- Investigate cryptotanshinone's biosynthesis pathways and related metabolic intermediates.
- Support research into plant secondary metabolism and biochemical profiling.
Cryptotanshinone Analysis Service Offered by Creative Proteomics
Cryptotanshinone Quantitative Analysis (Absolute & Relative)
- High-sensitivity quantification using LC-MS/MS
- Suitable for plant extracts, fermentation broths, and engineered systems
- Includes standard curve generation and validation (LOD < 0.5 ng/mL)
Tanshinone Family Profiling
- Simultaneous detection of multiple diterpenoid quinones including Tanshinone I, IIA, IIB, and Dihydrotanshinone
- Comparative quantification to assess compound ratios within the extract
Metabolic Pathway Intermediate Detection
- Targeted analysis of biosynthetic intermediates such as miltiradiene, ferruginol, and GGPP
- Supports studies on biosynthesis, metabolic flux, and pathway optimization
Cryptotanshinone Biosynthesis Pathway Mapping
- Integration of metabolite data with pathway databases (e.g., KEGG, PlantCyc)
- Visualization of biosynthetic logic and compound flow
Chemotype Differentiation & Comparative Analysis
- Profiling of different Salvia species or genetically modified lines
- Statistical comparison of Cryptotanshinone and related compounds across samples
Cell Line or Microbial Fermentation Monitoring
- Evaluation of engineered strains producing Cryptotanshinone
- Batch-to-batch consistency checks and yield optimization
Stability Testing Under Various Conditions
- Assessment of Cryptotanshinone stability under light, pH, temperature, and solvent exposure
- Data provided for formulation or storage studies
Targeted Metabolomics Reporting
- Quantitative data of related metabolites involved in the diterpenoid pathway
- Includes sample-to-sample variability, heatmaps, PCA plots, and pathway overlays
List of Detected Cryptotanshinone and Related Metabolites
| Category | Compound Name | Function / Relevance |
|---|---|---|
| Primary Analyte | Cryptotanshinone | Core compound of interest; diterpenoid quinone from Salvia miltiorrhiza |
| Tanshinone Family | Tanshinone I | Co-occurring diterpenoid with similar bioactivity |
| Tanshinone IIA | Major bioactive compound in Danshen; used for comparative profiling | |
| Tanshinone IIB | Oxidized analog; structural variant | |
| Dihydrotanshinone I | Reduced form of CT; analyzed for stability & biosynthetic insights | |
| Biosynthetic Intermediates | Geranylgeranyl diphosphate (GGPP) | Precursor in diterpene backbone synthesis |
| Miltiradiene | Immediate precursor to ferruginol in CT biosynthesis | |
| Ferruginol | Early-stage oxygenated intermediate | |
| Succinyl-CoA | Central intermediate in TCA cycle-linked biosynthesis | |
| 11-Hydroxyferruginol | Hydroxylated derivative; downstream precursor | |
| Oxidation Products | 15,16-Dihydrotanshinone | Metabolic or degradation product |
| Hydroxytanshinone IIA | Hydroxylated analog used in oxidative pathway tracing | |
| Pathway Markers | Abietic acid | Shared intermediate in labdane-type diterpenoid pathways |
| Dehydroabietic acid | Related oxidation product in diterpenoid metabolism | |
| Associated Pathways | MEP Pathway Metabolites (e.g. DXP, MEP) | Non-mevalonate pathway intermediates upstream of GGPP |
| Phenylpropanoid-derived Compounds | Co-regulated pathways; potential cross-talk with diterpenoid biosynthesis | |
| Shikimate Pathway Intermediates | Provides precursors for aromatic modification |
Advantages of Cryptotanshinone Assay
- Ultra-Low Detection Limit: Limit of Detection (LOD) for Cryptotanshinone: < 0.5 ng/mL using LC-MS/MS (QTRAP 6500+)
- High Quantitative Accuracy: Relative Standard Deviation (RSD): < 5% across biological replicates
- Exceptional Linearity: Calibration curve R²: > 0.999 over 3 orders of magnitude (0.5–1000 ng/mL)
- Rapid Turnaround Time: 96 samples processed within 72 hours (standard throughput mode)
- Wide Metabolite Coverage: Detects 25+ diterpenoid compounds, including biosynthetic intermediates and analogs
- Reproducibility Across Batches: Inter-day variability (CV): < 7%; Intra-day variability: < 5%
- Integrated Pathway Profiling: Pathway visualization and metabolite flux mapping using KEGG/PlantCyc-based overlays
- Flexible Sample Compatibility: Compatible with >6 biological matrices, including dry plant, extracts, cell pellets
- Method Validation Included: Each batch includes spike recovery (85–115%) and blank controls
- Data Delivery Format: Results provided in .raw, .xlsx, .pdf, and interactive pathway maps formats
Workflow for Cryptotanshinone Analysis Service
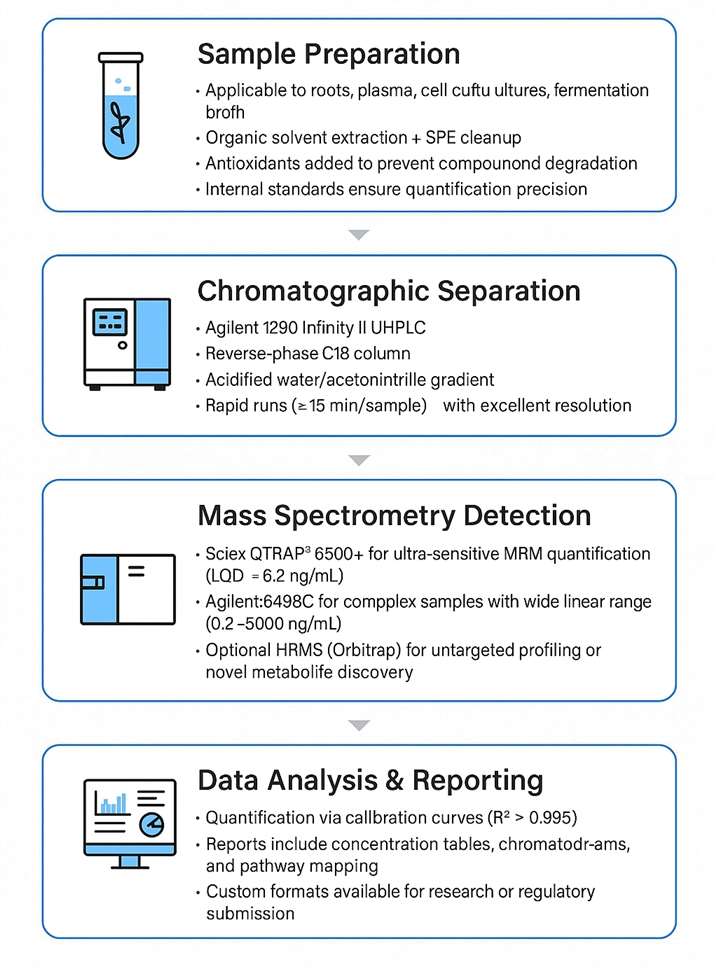
Technology Platform for Cryptotanshinone Analysis Service
Sciex QTRAP® 6500+ LC-MS/MS (MRM, IDA): Ultra-sensitive quantification (LOD < 0.5 ng/mL) and metabolite profiling.
Agilent 6495C Triple Quad High-precision LC-MS/MS: High-throughput quantitative analysis with excellent reproducibility (CV < 5%).
Agilent 1290 Infinity II UPLC / HPLC Separation: High-resolution separation of Cryptotanshinone and analogs; supports both MS and UV detection.

SCIEX Triple Quad™ 6500+ (Figure from Sciex)

Agilent 6495C Triple Quadrupole (Figure from Agilent)

Agilent 1260 Infinity II HPLC (Fig from Agilent)
Sample Requirements for Cryptotanshinone Analysis Service
| Sample Type | Minimum Amount Required |
|---|---|
| Fresh or Frozen Plant Tissue | ≥ 500 mg |
| Dried Botanical Material | ≥ 100 mg (finely ground) |
| Extracts (Ethanol, Methanol) | ≥ 200 µL |
| Fermentation Broth | ≥ 2 mL (unfiltered) |
| Cell Cultures (Pellet) | ≥ 1 x 10⁷ cells |
| Biological Fluids | ≥ 500 µL |
Applications of Cryptotanshinone Assay Service
Botanical Quality Control
Authenticate herbal materials and validate standardization levels.
Plant Metabolomics
Understand diterpenoid metabolic pathways in plant systems.
Synthetic Biology Monitoring
Validate cryptotanshinone production in engineered organisms.
Natural Product Research
Identify novel analogs and investigate biosynthetic logic.
Natural Product Research
Identify novel analogs and investigate biosynthetic logic.
Agronomic Phenotyping
Characterize chemotypes across Salvia cultivars and mutants.
FAQ of Cryptotanshinone Analysis Service
How should I prepare and ship my samples to ensure stability?
For plant tissues or fermentation broths, freeze samples at -80°C or ship on dry ice. Liquid samples can be stabilized with additives like 0.1% formic acid to prevent degradation. Light-sensitive samples should be packed in opaque containers.
What if my Cryptotanshinone concentration is too high or too low for detection?
Our LC-MS/MS method has a broad linear range (0.5–1000 ng/mL). For trace-level samples, we offer optional pre-concentration protocols (discuss during project setup).
Can you help interpret unexpected peaks or unidentified metabolites in my data?
Yes! Raw data files (.raw) are included in deliverables, and our standard report highlights anomalies. For deeper analysis (e.g., unknown metabolite identification), upgrade to our Expert Annotation Service for detailed insights from our metabolomics specialists.
Do you offer discounts for large-scale projects?
Yes! Volume discounts apply for projects with 50+ samples (tiered pricing available).
Can I customize the metabolite panel beyond the listed compounds?
Absolutely. For example, we can integrate cryptotanshinone with phenolic acids (e.g., salvianolic acid B) or other target metabolites.
Will you notify me if contaminants (e.g., microbial toxins) are detected?
Yes. We flag non-target peaks in your report and provide a complimentary Contaminant Screening Summary to help identify potential sample quality issues.
Do you support international sample shipments?
Yes. We accept global shipments and provide guidance on customs documentation, import permits, and temperature-controlled logistics.
Learn about other Q&A about other technologies.
Cryptotanshinone Analysis Service Case Study

Title: Simultaneous determination of danshensu, rosmarinic acid, cryptotanshinone, tanshinone IIA, tanshinone I and dihydrotanshinone I by liquid chromatographic–mass spectrometry and the application to pharmacokinetics in rats
Journal: Journal of Pharmaceutical and Biomedical Analysis
Published: 2010
- Background
- Materials & Methods
- Results
- Conclusion
- Reference
Salvia miltiorrhiza Bunge (Danshen) is widely used in traditional Chinese medicine for cardiovascular and liver-related diseases. Its therapeutic efficacy is mainly attributed to two classes of bioactive compounds: hydrophilic phenolic acids (e.g., danshensu, rosmarinic acid) and lipophilic diterpenoid quinones (e.g., cryptotanshinone, tanshinone IIA). Due to their chemical differences and pharmacological relevance, it is critical to quantify both compound groups simultaneously in biological matrices for pharmacokinetic and quality control purposes. However, previous methods lacked comprehensive, sensitive quantification for both classes in vivo. This study developed and validated an LC–MS/MS method using electrospray ionization in both positive and negative modes for simultaneous quantification of six key constituents in rat plasma after oral administration of Danshen preparations.
Chemicals and Reagents
- Standards: Danshensu (DS), rosmarinic acid (RA), cryptotanshinone (CT), tanshinone I (TI), dihydrotanshinone I (DT), tanshinone IIA (TS), and fenofibrate (internal standard, I.S.).
- Solvents: Acetonitrile, ethyl acetate, glacial acetic acid (HPLC grade), hydrochloric acid.
Animals
- Male rats (specific pathogen free) used for plasma collection and pharmacokinetic studies.
- All procedures followed institutional animal care guidelines.
Sample Preparation
- Liquid–liquid extraction chosen over organic precipitation.
- Optimal condition: Acidification to pH 2.0 with 12% HCl, followed by double extraction with ethyl acetate.
- Supernatants were evaporated and reconstituted before LC–MS/MS analysis.
Chromatographic Conditions
- Column: Capcell Pak MG C18.
- Mobile phase: Gradient elution from acetonitrile–0.5% acetic acid aqueous solution (8:92 to 78:22, v/v).
- Flow rate: 0.2 mL/min.
- Retention times for DS, RA, CT, DT, TI, TS, and I.S. ranged from ~4.35 to 25.26 min.
Mass Spectrometry Conditions
- Ionization: Electrospray ionization (ESI).
- Mode: Negative ion mode for phenolic acids (first 19 min), then switched to positive mode for tanshinones.
- MRM (Multiple Reaction Monitoring) used for quantification.
- Instrumental parameters (temperature, flow rate, fragment energy) optimized for each analyte.
Method Validation
- Parameters evaluated: Selectivity, linearity, LOD/LOQ, precision, accuracy, recovery, and stability.
- Calibration performed over a range of 0.1–2500 ng/mL depending on the analyte.
- Stability assessed under various storage and processing conditions.
Pharmacokinetic Study
- Rats administered single oral dose of danshen extract.
- Plasma samples collected at multiple time points.
- Concentration–time profiles analyzed using a two-compartment model.
A sensitive and selective LC–ESI–MS/MS method was successfully developed and validated for the simultaneous determination of six active compounds (danshensu, rosmarinic acid, cryptotanshinone, tanshinone I, dihydrotanshinone I, and tanshinone IIA) from danshen extracts in rat plasma. Optimal sample preparation involved liquid–liquid extraction at pH 2.0 using ethyl acetate. A Capcell Pak MG C18 column and a gradient elution system (acetonitrile–0.5% acetic acid) enabled efficient separation. Electrospray ionization in time-segmented negative/positive ion modes improved sensitivity for both phenolic acids and tanshinones. The method exhibited good linearity (r² > 0.995), acceptable LOD and LOQ, high extraction recoveries (>60%), and stable performance under various storage and handling conditions. Pharmacokinetic analysis showed all compounds followed a two-compartment open model, with notable differences in absorption, distribution, and elimination. TS was shown to be both a parent compound and a metabolite of CT.
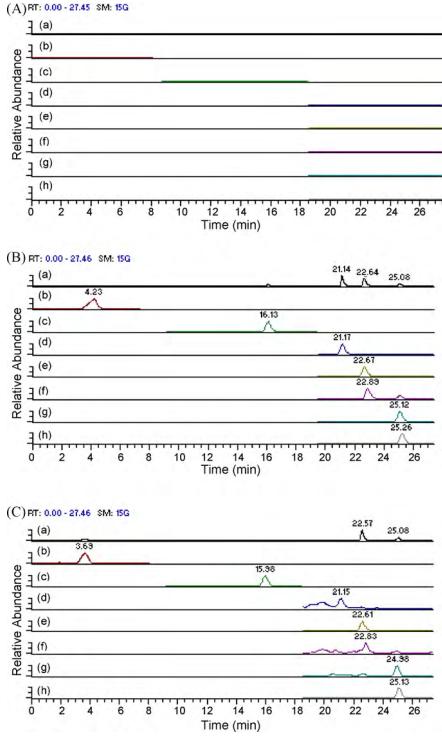 LC–MS/MS chromatograms of: (A) blank rat plasma; (B) blank plasma spiked with six active compounds and I.S.; (C) rat plasma at 1.5 h after oral administration of danshen extract. Panels show total ion current (a) and selected reaction monitoring (SRM) for each analyte: danshensu (b), rosmarinic acid (c), cryptotanshinone (d), dihydrotanshinone I (e), tanshinone I (f), tanshinone IIA (g), and internal standard fenofibrate (h).
LC–MS/MS chromatograms of: (A) blank rat plasma; (B) blank plasma spiked with six active compounds and I.S.; (C) rat plasma at 1.5 h after oral administration of danshen extract. Panels show total ion current (a) and selected reaction monitoring (SRM) for each analyte: danshensu (b), rosmarinic acid (c), cryptotanshinone (d), dihydrotanshinone I (e), tanshinone I (f), tanshinone IIA (g), and internal standard fenofibrate (h).
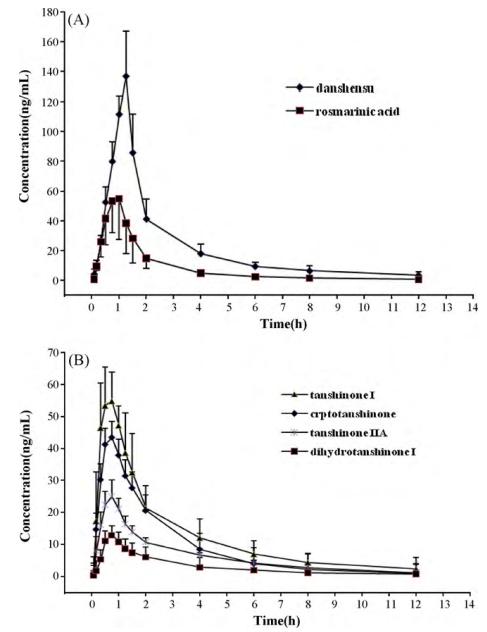 Pharmacokinetics profiles of: (A) danshensu and rosmarinic acid; (B) cryptotanshinone, tanshinone I, dihydrotanshinone I and tanshinone IIA in plasma following administration of a single oral dose of danshen extracts to rats.
Pharmacokinetics profiles of: (A) danshensu and rosmarinic acid; (B) cryptotanshinone, tanshinone I, dihydrotanshinone I and tanshinone IIA in plasma following administration of a single oral dose of danshen extracts to rats.
The developed LC–ESI–MS/MS method is accurate, precise, sensitive, and robust, making it suitable for the simultaneous quantification and pharmacokinetic profiling of key phenolic and diterpenoid compounds in danshen extracts. This study provides a comprehensive pharmacokinetic understanding essential for the clinical and therapeutic evaluation of danshen preparations.
Reference
- Liu, Yun, et al. "Simultaneous determination of danshensu, rosmarinic acid, cryptotanshinone, tanshinone IIA, tanshinone I and dihydrotanshinone I by liquid chromatographic–mass spectrometry and the application to pharmacokinetics in rats." Journal of Pharmaceutical and Biomedical Analysis 53.3 (2010): 698-704. https://doi.org/10.1016/j.jpba.2010.03.041
Publications
Here are some publications in Metabolomics research from our clients:

- High Levels of Oxidative Stress Early after HSCT Are Associated with Later Adverse Outcomes. 2024. https://doi.org/10.1016/j.jtct.2023.12.096
- A personalized probabilistic approach to ovarian cancer diagnostics. 2024. https://doi.org/10.1016/j.ygyno.2023.12.030
- The activity of the aryl hydrocarbon receptor in T cells tunes the gut microenvironment to sustain autoimmunity and neuroinflammation. 2023. https://doi.org/10.1371/journal.pbio.3002000
- Fatty Acid and Antioxidant Profile of Eggs from Pasture-Raised Hens Fed a Corn- and Soy-Free Diet and Supplemented with Grass-Fed Beef Suet and Liver. 2022. https://doi.org/10.3390/foods11213404
- Anti-inflammatory activity of black soldier fly oil associated with modulation of tlr signaling: A metabolomic approach. 2023. https://doi.org/10.3390/ijms241310634







