Serotonin (5-hydroxytryptamine, 5-HT) is a monoamine neurotransmitter and modulator widely distributed in the central nervous system, intestinal tract and peripheral tissues. Its metabolism and functional regulation involve complex molecular networks, and are closely related to physiological processes such as emotion, cognition, digestion and immunity. Its disorder can lead to depression, anxiety and other mental diseases. It plays an important role in human physiological and psychological health. This article explores serotonin's receptor subtypes, metabolic pathways, and physiological roles in health and medical application.
Summary of Serotonin
Serotonin (5-hydroxytryptamine) is an indoleamine compound synthesized from tryptophan through hydroxylation and decarboxylation. Although constituting merely 2% of the body's total serotonin, the cerebral portion governs crucial neuromodulatory functions. This molecule acts as a conductor of a symphony orchestra, coordinating neural activities across brain regions through activation of 5-HT1A to -HT7 receptor families.
Receptor subtypes of serotonin and their functional differentiation
Serotonin (5-HT) achieves complex physiological regulation through 14 receptor subtypes, which are structurally categorized into 7 classes (5-HT1-7). These receptors encompass two major types – G protein-coupled receptors (GPCRs) and ligand-gated ion channels – forming a sophisticated functional division system.
(1) 5-HT1 (Gi/o-coupled)
5-HT1A: Densely expressed in raphe nuclei (autoreceptors) and hippocampus. Activation inhibits adenylate cyclase (AC), reducing cAMP levels. It can modulate anxiety, depression, and neurogenesis.; 5-HT1B/D: Primarily located in the cortex and cerebral vasculature. Suppress voltage-gated calcium channels; clinically targeted by triptans for migraine treatment.
(2) 5-HT2 (Gq/11-coupled)
5-HT2A: Predominantly expressed in the prefrontal cortex. Activates phospholipase C (PLC) to generate IP3/DAG. Mediates hallucinogenic effects (e.g., LSD) and antidepressant neuroplasticity, with receptor activation shown to increase dendritic spine density by 8.3%; 5-HT2C: Hypothalamus-enriched. Regulates appetite and energy metabolism. Aberrant mRNA editing of this receptor correlates with obsessive-compulsive disorder (OCD).
(3) 5-HT3 (ionotropic type)
The sole ligand-gated cation channel mediates rapid (<10 ms) neurotransmission. Activation induces Na+/Ca2+ influx, directly triggering emetic reflex.
(4) 5-HT4/5/6/7 (Gs-coupled)
5-HT4: Enhances hippocampal acetylcholine release, improving memory encoding efficiency by 30%; 5-HT6: Antagonists ameliorate cognitive deficits in Alzheimer's disease models; 5-HT7: Regulates circadian rhythm, with its genetic polymorphisms showing significant associations with sleep disorders.
Select Service
Physiological Functions of Serotonin
Serotonin is a signaling molecule with dual neurotransmitter and hormonal properties. Its actions span the central nervous system and peripheral organs, establishing a multi-tiered physiological regulatory network.
Central nervous system regulation
(1) Emotional and behavioral homeostasis
Serotonergic neurons in the raphe nuclei project to the prefrontal cortex and limbic system, suppressing amygdala hyperactivation (reducing anxiety responses by 35%) while enhancing hippocampal neurogenesis (15% increase in newborn neuron survival). Social behavior experiments show that elevated 5-HT release in the mouse raphe nuclei increases prosocial interaction frequency by 40%.
(2) Cognition and learning memory
Prefrontal 5-HT1A receptor activation enhances working memory precision, while 5-HT4 receptors promote long-term potentiation (LTP) via the cAMP-PKA signaling pathway, enhancing spatial memory formation efficiency by 25%. fMRI studies confirm a positive correlation (r=0.62) between 5-HT levels and default mode network functional connectivity strength in healthy subjects.
(3) Sleep-wake cycle
Serotonergic inputs to the suprachiasmatic nucleus inhibit melatonin secretion, maintaining daytime wakefulness. During non-REM sleep, raphe nuclei neuronal firing rates drop to 1-2 Hz, facilitating slow-wave sleep (50% δ-wave power enhancement).
Peripheral system function
(1) Digestive system regulation
Enterochromaffin cells in the gut produce 95% of the body's 5-HT. This serotonin activates intestinal 5-HT3/4 receptors to accelerate peristalsis and stimulates vagus nerve afferent fibers, transmitting satiety signals to the nucleus tractus solitarius。
(2) Coagulation and vascular tension
Platelets uptake and store blood 5-HT in dense granules. Upon vascular injury, released 5-HT induces vasoconstriction via 5-HT2A receptors while enhancing platelet aggregation.
(3) Bone metabolism and thermoregulation
Osteoblastic 5-HT1B receptors inhibit bone formation, while 5-HT2B receptor activation promotes osteoclast differentiation. Hypothalamic serotonergic signaling regulates brown adipose thermogenesis, maintaining core body temperature fluctuations within ≤ 0.5°C.
Cross-system interaction
(1) Immunomodulation
Monocytic 5-HT1E receptor activation inhibits IL-6 secretion, reducing inflammatory cytokine levels by 40%. Mast cell-derived 5-HT enhances vascular permeability via 5-HT2A receptors.
(2) Neuroendocrine axis
Hypothalamic 5-HT neurons stimulate CRH release via 5-HT1A receptors; this activates the HPA axis to fine-tune stress responses.
(3) Intergration of energy metabolism
Pancreatic β-cell 5-HT3 receptors regulate pulsatile insulin secretion; adipose tissue 5-HT2A receptors suppress lipolysis.
Metabolic Pathways of Serotonin
The metabolism of serotonin (5-HT) includes synthesis, release, reuptake and decomposition.
The synthesis of serotonin
Serotonin is synthesized using the essential amino acid tryptophan as a precursor. After entering the central nervous system through the blood-brain barrier, it is completed by a two-step enzymatic reaction in the cytoplasm of neurons: First, tryptophan hydroxylase (TPH, containing both TPH1 and TPH2 subtypes, of which TPH2 is specifically expressed in central neurons) catalyzes the hydroxylation of tryptophan to 5-hydroxytryptophan (5-HTP) in a synthetic rate-limiting step that requires tetrahydrobiopterin (BH4) as a cofactor and depends on the participation of iron ions (Fe² +) and oxygen molecules.
Subsequently, aromatic amino acid decarboxylase (AADC) converts 5-HTP decarboxylase to 5-HT with vitamin B6 (pyridoxal phosphate) as a coenzyme. The synthesized 5-HT is actively transported to the synaptic vesicles for storage via vesicular monoamine transporter (VMAT2) to avoid the degradation of monoamine oxidase (MAO) in the cytoplasm. TPH activity is regulated by gene expression (e.g., TPH2 promoter polymorphism), tryptophan availability (influenced by dietary intake and insulin-regulated plasma tryptophan levels), and negative feedback (5-HT inhibits TPH transcription by activating the presynaptic 5-HT1A autoreceptor).
In addition, abnormalities in the BH4 synthesis pathway (involving GTP cyclohydrolase I, etc.) can lead to dyssynthesis of 5-HT, which is associated with depression, anxiety, and Parkinson's disease, while deficiencies in AADC function may lead to rare monoamine metabolic disorders. Clinically, TPH inhibitors (such as p-chlorophenylalanine) and 5-HTP supplements are used to study or treat disorders related to the 5-HT-energy system.
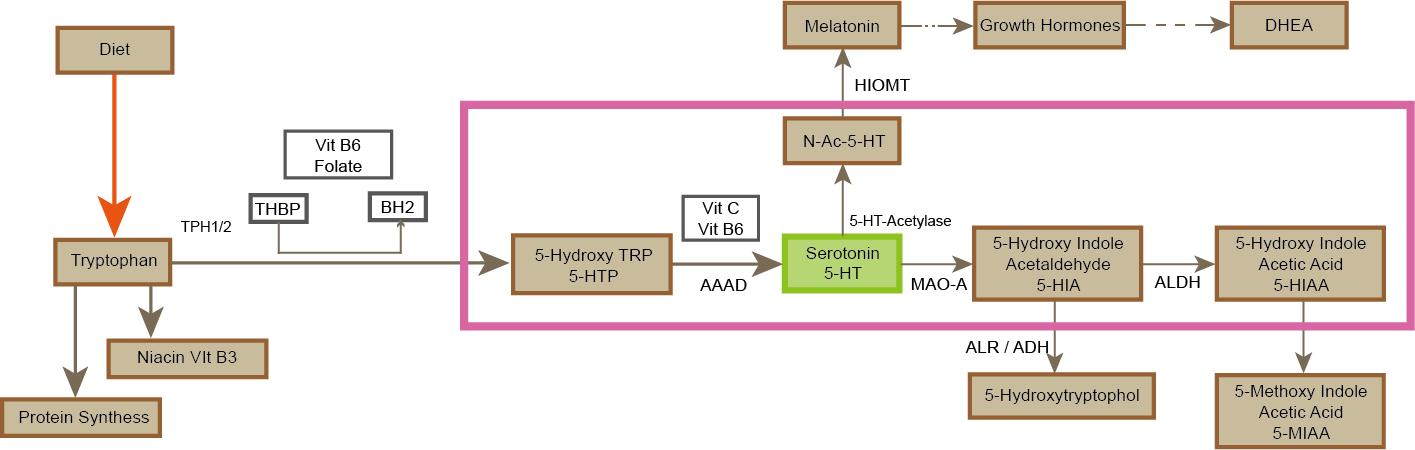 Figure3.The metabolic pathways of serotonin.
Figure3.The metabolic pathways of serotonin.
The release of serotonin
The release of serotonin begins with the activation of the action potential of neurons in the raphe nucleus, triggered by the opening of voltage-gated calcium channels such as type N, P/Q and an increase in intracellular Ca²+ concentration, which induces the fusion of synaptic vesicles with the presynaptic membrane via SNARE protein complexes. Quantized release of 5-HT stored in vesicles into the synaptic cleft; The release process is regulated by multiple levels: The presynaptic 5-HT1B/D autoreceptor inhibits adenylate cyclase and calcium channel activities through Gi/o protein, and negative feedback reduces the release of the transmitter. Then, heterologous receptors, such as glutamate AMPA receptor and GABA_B receptor, indirectly regulate the release intensity by regulating neuronal excitability. Neuromodulators (such as oxytocin and corticotrophin releasing factor) enhance vesicular mobilization efficiency by activating intracellular PKC/PKA signaling pathways.
In addition, there are two modes of 5-HT release: phasic and tonic: the former is triggered by high-frequency cluster discharge, mediating rapid signal transmission (such as stress response); The latter is maintained by low frequency continuous discharge and regulates basal neural activity (such as emotional homeostasis) in the distal brain region through volume transmission. The dynamics of release are influenced by the spontaneous discharge rhythm of raphe nucleus neurons and by the regulation of astrocytes on the synaptic cleft K+ /pH by abnormalities, which can lead to dysfunction of the prefrontal limbic system circuit, which is closely related to pathological phenotypes such as depression, anxiety, and obsessive-compulsive disorder.
The reuptake of serotonin
The reuptake of serotonin is mainly mediated by the 5-HT transporter (SERT, the transmembrane protein encoded by the SLC6A4 gene) on the presynaptic membrane, which re-ingest the inverse concentration difference of 5-HT in the synaptic cleft into the cytoplasm through an active transport mechanism dependent on and Na+/Cl- gradient; The 12 transmembrane domains of SERT form substrate-binding pockets which undergo a conformational change after extracellular Na+/Cl- combination that induces 5-HT to bind to the transporter and transport to the intracellular level, followed by intracellular K+ reverse transport to complete the cycle; The efficiency of reuptake is dynamically regulated by the level of SERT expression, local ion gradients in the presynaptic membrane and phosphorylation modifications.
In addition, the presynaptic 5-HT1B/D autoreceptor inhibits the cAMP-PKA signaling pathway through Gi/o protein, indirectly reducing SERT membrane expression and reuptake activity. SERT expressed by astrocytes is also involved in extrapsynaptic 5-HT clearance. Reuptake abnormalities can lead to a sustained increase or depletion of 5-HT in the synaptic gap, which may induce 5-HT syndrome (overactivation of 5-HT2A receptors), depression (insufficient prefrontal 5-HT transmission) and obsessive-compulsive disorder (OCD). Selective 5-HT reuptake inhibitors (SSRIs) extend the duration of 5-HT synaptic action by competitively binding to the bottom sites of SERTs (such as exon 1b domain), blocking their transport function, but long-term administration can induce SERT endocytosis or down-regulated expression, leading to drug tolerance. This process, together with the secondary storage of 5-HT by vesicle monoamine transporter (VMAT2) and the degradation of monoamine oxidase (MAO) in mitochondrial outer membrane, maintains the dynamic balance of 5-HT neurotransmission.
Degradation of serotonin
The degradation of serotonin is mainly mediated by MAO, mainly MAO-A and cofactor is FAD in the outer membrane of mitochondria catalyzes the oxidative deamination of free 5-HT in the cytoplasm or synaptic cleft to produce 5-hydroxyindoleacetaldehyde, which is then rapidly transformed by aldehyde dehydrogenase (ALDH) into the final product 5-hydroxyindoleacetic acid (5-HIAA) and excreted by the kidney. This process is influenced by MAO activity (regulated by MAOA gene polymorphisms, hormone levels, and MAO inhibitors), substrate availability (SERT reuptake efficiency determines the amount of degraded substrate), and mitochondrial function, where abnormalities (such as MAO-A overactivation or SERT deficiency) can lead to imbalances in synaptic 5-HT concentration. It is associated with depression, aggressive behavior, and 5-HT syndrome, and 5-HIAA level is also used as a biomarker for clinical assessment of 5-HT metabolic status.
Abnormal regulation of serotonin
Abnormal serotonin (5-HT) regulation is closely related to a variety of psychiatric phenotypes: reduced synthesis or abnormal reuptake of presynaptic transmitters (such as SERT gene polymorphism) can lead to functional imbalance of the prefrontal cortical-limbic system circuit, leading to depression (anhedonia, low mood) and anxiety disorders (hypervigilance); Low function of 5-HT1A receptor is associated with over-activation of amygdala, aggravating fear memory generalization and post-traumatic stress disorder. Overactivation of 5-HT2A receptor may induce positive symptoms of schizophrenia (hallucinations, delusions) through dysregulation of glutaminergic system; In addition, dystonoregulation of 5-HT can impair the decision-making function of the accumben-striate reward circuit, leading to obsessive-compulsive disorder (repetitive behavior) and impulse control disorders. Together, these phenotypes reflect the key regulatory role of the serotonin system in the dynamic coding of neural circuits (such as synaptic plasticity, neurogenesis) and the multidimensional behavioral deficits caused by abnormalities [1].
Select Service
Serotonin Metabolism and Health
Serotonin metabolism and mental health
Depression
Decreased 5-HT input in the prefrontal cortex (PFC) leads to atrophy of the dendritic spines of glutaminergic pyramidal neurons, while ventral tegmental area (VTA) dopaminergic neurons are inhibited by 5-HT2C receptor activation, resulting in defective reward circuit function. The delayed effect of SSRIs is due to presynaptic 5-HT1A autoreceptor desensitization: long-term administration down-regulates the GI-coupled G protein subunits (such as Giα3), deactivates the AC-cAMP-PKA pathway, and ultimately enhances TPH2 transcription and vesicular release. Studies have shown that higher dietary histidine intake (1400 mg/day) is associated with lower obesity rates and body mass index (BMI), and that in overweight and obese individuals, histidine supplementation improves insulin resistance, an important feature of metabolic syndrome. In addition, histidine affects appetite and metabolic rate by increasing histamine levels and histamine decarboxylase activity in the hypothalamus, which may have a positive effect on weight management.
Anxiety disorder:
Insufficient expression of 5-HT1A receptor in the basolateral nucleus of amygdala (BLA) leads to decreased inhibition of GABAergic interneurons and overactivation of glutamatergic projection neurons, leading to conditioned fear generalization; Increased 5-HT reuptake further exacerbates 5-HT depletion, and SSRIs work by blocking SERT and promoting BDNF-TrkB mediated synaptic remodeling.
Schizophrenia:
Prefrontal 5-HT2A receptor and glutamate NMDA receptor function antagonism in postsynaptic membrane formation, 5-HT2A overactivation inhibits NMDA receptor current, resulting in abnormal gamma oscillations and positive symptoms; Negative symptoms are associated with reduced 5-HT input from the raphe nucleus to the nucleus accumbens (low activity of 5-HT1A receptor), and some atypical antipsychotics improve symptoms by antagonizing the 5-HT2A/2C receptor.
Obsessive-compulsive disorder:
Overactivation of striatum 5-HT2C receptor enhances D1-MSN activity and promotes repetitive behaviors; Deep brain stimulation (DBS) targeting the ventral internal capsule improves symptoms by inhibiting 5-HT dystonia [2].
Effects of nutrition and lifestyle on serotonin metabolism
Nutrient regulation:
The rate of tryptophan into the brain is regulated by insulin - carbohydrates stimulate insulin secretion, promote branched-chain amino acids (BCAAs) into the muscle, indirectly increase the tryptophan/BCAA ratio, and increase the synthetic substrate in the brain. Vitamin B6 is an AADC coenzyme, and its deficiency can lead to 5-HTP accumulation and 5-HT deficiency. Iron is an essential cofactor of TPH2, and iron deficiency (such as menorrhagia in women) directly inhibits 5-HT production, which is associated with premenstrual dysphoria disorder (PMDD). Intestinal flora metabolizes dietary fiber to produce SCFAs such as propionic acid and butyric acid, activate the free fatty acid receptor FFAR2 on the surface of intestinal EC cells, promote the expression of TPH1 and the release of 5-HT, and then regulate central emotions through the afferent signals of vagus nerve.
Environmental and behavioral intervention:
Strong light irradiation (>10,000 lux) is projected to the suprachiasmatic nucleus of hypothalamus through retinal ipRGC cells, which activates the firing of 5-HT neurons in raphe nucleus, improves TPH2 activity and 5-HT synthesis; Aerobic exercise enhances TPH2 gene expression by activating histone deacetylase (HDAC) inhibitors through the release of beta-hydroxybutyric acid from muscles. Chronic stress inhibited TPH2 promoter activity through glucocorticoid receptor signaling, upregulated MAO-A expression, and doubly decreased 5-HT levels.
Medical application of drug intervention and metabolic regulation
SSRIs (such as sertraline) selectively bind to the substrate pocket of SERT (exon 1b domain), blocking reuptake and inducing SERT entosis (via β-arrestin signaling); Tricyclic antidepressants (such as amitriptyline), in addition to inhibiting SERT, also antagonize histamine H1 and muscarine receptors, causing sedation and anticholinergic side effects. MAO inhibitors (such as antamphetamine) irreversibly bind to the FAD cogroup of MAO-A to increase 5-HT and NE levels, but tyramine intake needs to be limited to avoid hypertensive crises.
Partial agonists of 5-HT1A receptor (such as tandospirone) reduce Gi protein dissociation by stabilizing receptor conformation, enhance signal transduction efficiency, and rapidly relieve generalized anxiety. 5-HT2A reverse agonists (such as Pimovan serin) are more effective in controlling schizophrenic hallucinations by inhibiting basal activity rather than antagonizing ligand activation. The multimodulated drug votioxetine simultaneously inhibits SERT and 5-HT3 receptors (antagonizing gastrointestinal side effects) and activates 5-HT1A receptors to enhance antidepressant response.
Combined detection of 5-HTTLPR polymorphism of SLC6A4 gene (S allele carriers have poor response to SSRIs) and FKBP5 genotype (glucocorticoid signaling regulation) can optimize drug selection. The hallucinogenic drug Psilocybin triggers mTORC1 signaling by activating the 5-HT2A receptor, rapidly reestablishing prefrontal limbic system synaptic connections, and its antidepressant effects can last for weeks with a single dose.
Conclusion
Serotonin is a small monoamine derived from tryptophan, whose functions span the nervous, endocrine, and immune systems. It is very important for human health, both physical and mental. The complexity of the serotonin metabolic network is regulated at multiple scales: at the genetic level (e.g., TPH2 rs4570625 polymorphism affects transcription factor Pax6 binding), at the epigenetic level (MAOA gene methylation is associated with childhood trauma), and at the systemic level (gut-brain axis bidirectional communication).
Future studies need to integrate induced pluripotent stem cell (iPSC) models (to simulate the deficiency of 5-HT metabolism in patient-specific neurons) with in vivo calcium imaging techniques (to monitor the activity of raphe nucleus neuron clusters in real time) to analyze the individualized pathological mechanisms. Therapeutic strategies will tend towards multi-target synergistic interventions: for example, combining SSRIs with 5-HT4 receptor agonists (promoting hippocampal neurogenesis) or anti-inflammatory agents (inhibiting microglial activation).
In addition, intestinal flora transplantation (FMT) may be an adjunct therapy for depression by restoring the abundance of SCFAs producing bacteria to increase peripheral 5-HT levels. Public education needs to emphasize nutritional balance (tryptophan/vitamin B6/ iron co-supplementation), light management (30 minutes daily morning light), and stress regulation (mindfulness meditation reduces cortisol) to maintain 5-HT metabolic homeostasis from a lifestyle dimension. Ultimately, interdisciplinary integration (neuroscience, metabolomics, and digital health technologies) will lead to the establishment of precise prevention and treatment systems for serotonin-related diseases.
References
- O' Mahorry, S.M., Clarke, G., Borre, Y.E., Dinan, T.G., Cryan, J.F., 2015. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behavioural Brain Resaerch. 277, 32-48. https://doi.org/10.1016/J.BBR.2014.07.027.
- Marcinkiewcz, C.A., Mazzone, C.M., D' Agostino, G., Halladay, L.R., Hardaway, J.A., Diberto, J.F., Navarro, M., Burnham, N., Cristiano, C., Dorrier, C.E., Tipton, G.J., Ramakrishnan, C., Kozicz, T., Deisseroch, K., Thiele, T.E., McElligott, Z.A., Holmes, A., Heister, L.K., Kash, T.L., 2016. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature. 537, 97-101. https://doi.org/10.1038/NATURE19318.




