What are the properties of tryptophan?
As an essential amino acid, tryptophan exhibits multiple functional properties in biological systems with its unique indole ring structure. The indole group of its side chain is not only the key hydrophobic unit for the three-dimensional folding of proteins, but also endows the bioactive diversity of its metabolites. As the only essential amino acid containing aromatic heterocyclic rings, tryptophan cannot be synthesized by mammals independently, but needs to be ingested through diet. Its metabolic network is distributed in the intestines, liver, brain and other organs, forming a cross-tissue cooperative system. Tryptophan is functionally differentiated through three core pathways: In the 5-hydroxytryptophan (5-HT) pathway, tryptophan hydroxylase catalyzes the production of the "happy hormone" 5-HT, which regulates mood, sleep and intestinal movement; Kynurenine pathway is activated by IDO/TDO rate-limiting enzyme, producing NAD+ to maintain cell energy homeostasis, and generating kynurenine and other immunomodulatory molecules, which affect T cell differentiation through aromatics receptor (AhR); About 5% of tryptophan is metabolized into indole compounds by intestinal flora, which not only strengthens intestinal barrier function, but also acts as signaling molecules to regulate systemic inflammatory response. This metabolic shunt mechanism makes tryptophan a hub connecting nervous, immune and metabolic systems. The disruption of its homeostasis is closely related to depression, Alzheimer's disease and tumor immune escape, which highlights its research value in precision medicine.
Tryptophan is involved in three major metabolic pathways
Tryptophan achieves diversified expression of its biological functions through three metabolic pathways: In 5-hydroxytryptophan (5-HT) synthesis pathway, tryptophan hydroxylase (TPH) catalyzed the production of neurotransmitter 5-HT. Brain TPH2 plays a leading role in emotional and cognitive regulation, while intestinal TPH1 is involved in gastrointestinal motility and gut-brain axis signal transmission; The kynurenine pathway (KP) is initiated by indoleamine 2, 3-dioxygenase (IDO) or tryptophan 2, 3-dioxygenase (TDO), which decomposes tryptophan into kynurenine. In this pathway, NAD+ is synthesized by the quinolinic acid branch to maintain cell energy homeostasis, and immunoregulatory molecules (such as kynurenic acid) are generated to activate aromatics receptor (AhR). Regulates Th17/Treg cell homeostasis; About 5% of tryptophan is metabolized by the gut flora into indole and its derivatives (such as indole-propionic acid), which strengthen intestinal barrier function by activating AhR and pregnane X receptors (PXR) and regulate systemic inflammatory responses. Three major pathways form a dynamic competitive network: 5-HT synthesis is severely restricted by the blood-brain barrier, KP pathway is significantly enhanced in the inflammatory or tumor microenvironment, and microbial metabolism influences host metabolic flow through "metabolic interception".
Tryptophan core function
Tryptophan is a multifunctional hub molecule in living systems. Its core functions are embodied in three dimensions: nervous regulation, immune balance and metabolic integration. In the nervous system, tryptophan is the only precursor of 5-hydroxytryptamine (5-HT). This neurotransmitter not only regulates mood, sleep and cognitive functions, but also influences gastrointestinal motility and appetite regulation through gut-brain axis communication mediated by enterochromaffin cells. Immunologically, kynurenine pathway metabolites act through a dual mechanism: On the one hand, the biosynthesis of NAD+ maintains cell energy homeostasis to support immune cell activation; on the other hand, molecules such as kynurenic acid activate aromatic receptor (AhR) to finely regulate Th17/Treg cell differentiation balance, acting as a "metabolic checkpoint" in autoimmune diseases and tumor microenvironment; The metabolic integration function is manifested as a cross-organ resource allocation strategy, the liver preferentially directs tryptophan to NAD+ synthesis to cope with energy crisis, while the indole compounds (such as indole propionic acid) produced by the intestinal flora through metabolic interception can not only enhance the intestinal epithelial barrier function, but also systematically inhibit inflammation. This functional polymorphism is due to the spatio-temporal specific regulation of metabolic pathways: brain tissue sequestrates 5-HT synthesis through the blood-brain barrier, inflammatory microenvironment induces IDO1 overexpression to remodel immune metabolic pattern, and bacterial metabolism dynamically regulates host availability of tryptophan pool. The interaction imbalance of the three functional networks is directly related to the monoamine hypothesis of depression, the neurotoxicity theory of quinolinic acid in Alzheimer's disease and the metabolic drug resistance mechanism of tumor immunotherapy, making tryptophan metabolism a focus of interdisciplinary research.
Select Service
Absorption, transport and uptake of tryptophan
Absorption of tryptophan: Obtaining essential amino acids from the outside world
Tryptophan is an essential amino acid, and its absorption process begins with dietary intake. In the gastrointestinal tract, food proteins are first decomposed into oligopeptids and free amino acids by pepsin and trypsin, and then free tryptophan is absorbed by a specific transport system in the upper membrane of small intestinal epithelial cells. This process is mainly mediated by sodium ion dependent neutral amino acid transporters, and its transport efficiency is affected by intestinal microenvironment pH and co-existing amino acids. About 95% of the absorbed tryptophan enters the liver through the portal vein, part of which is metabolized by the kynurenine pathway in the liver, and the rest enters the systemic circulation and distributes to the peripheral tissues. It is worth noting that about 5-10% of dietary tryptophan is not directly utilized by the host, but is metabolized by the intestinal flora in the end of the ileum through tryptophanase and decarboxylase into indoles, tryptamine and other derivatives. Such microbial metabolism not only reduces the total amount of tryptophan available to the host, but also activates aromatic receptor (AhR) to regulate intestinal barrier function. The absorption efficiency is dynamically regulated by many factors. This fine regulatory network of absorption and metabolism ensures the precise distribution of tryptophan in neurotransmitter synthesis, immune regulation and energy metabolism.
The homeostasis of tryptophan in the blood
The dynamic balance of tryptophan in blood is controlled by multiple organs. About 90-95% of dietary tryptophan absorbed through the small intestine is transported bound to albumin, and only 5% is present in the free form, and the ratio of this bound/free state determines its tissue availability. The liver, as the main metabolic organ, converts part of tryptophan into kynurenine by TDO enzyme (accounting for 60-70% of the total metabolic flux in the blood), and the rest is distributed to the brain, intestine and other tissues by the circulatory system. LAT1 transporter on blood-brain barrier endothelial cells selectively mediates the entry of free tryptophan into the brain and preferentially guarantees the synthesis of 5-hydroxytryptophan. In peripheral tissues, inflammatory factors induce IDO1 expression, directing tryptophan to immune-regulatory metabolites production. The maintenance of homeostasia depends on multiple feedback mechanisms: kynuridine pathway product NAD+ inhibits IDO1 transcription through SIRT1 deacetylase; Serotonin inhibits TPH enzyme activity by activating 5-HT1A autoreceptor negative feedback. In addition, the intestinal flora continuously metabolizes tryptophan to produce indole derivatives, which enter the liver through portal vein circulation for sulfation modification, forming a systematic metabolic buffer pool. This fine regulatory network stabilizes the blood tryptophan concentration at 50-100 μM, ensuring a balance between neurotransmitter synthesis, immune response and energy metabolism needs.
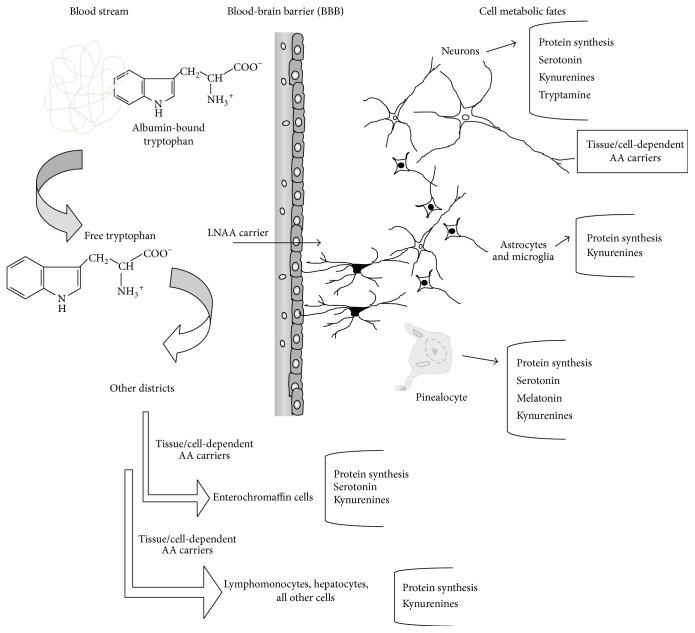 Figure 1. Tryptophan uptake to tissues and main metabolic destinies in either physiological or pathological states. (Palego L 2016)
Figure 1. Tryptophan uptake to tissues and main metabolic destinies in either physiological or pathological states. (Palego L 2016)
Tryptophan core metabolic pathways
Kynurenine pathway
The kynurenine pathway of tryptophan, a core branch of its metabolic network, is initiated by indoleamine 2, 3-dioxygenase (IDO) or tryptophan 2, 3-dioxygenase (TDO) to oxidize tryptophan to N-formylkynurenine in response to inflammatory factors or glucocorticoid stimulation. This pathway produces two major functional branches through a cascade reaction: the quinolinic acid branch eventually synthesizes NAD+, which provides key coenzymes for cellular energy metabolism; Metabolites such as kynurequinolinic acid, as aromatic receptor (AhR) ligands, participate in immune tolerance by regulating Th17/Treg cell balance. This pathway has dynamic plasticity -- in the tumor microenvironment, IDO1 overactivation leads to tryptophan depletion and accumulation of immunosuppressive molecules, promoting T cell dysfunction; In the nervous system, excessive accumulation of quinolinic acid can overactivate NMDA receptors, triggering glutamergic excitatory toxicity, which is associated with Alzheimer's disease pathology. Its dual properties (energy supply and immune regulation) make this pathway an important target for cancer immunotherapy (such as IDO inhibitors) and intervention in neurodegenerative diseases, but the redundancy of its metabolic network also poses clinical translational challenges.
The serotonergic pathway
The tryptophan-5-hydroxytryptophan (serotonin) pathway is one of the important metabolic pathways of tryptophan, which is mainly carried out in the central nervous system and the intestine. First, tryptophan is converted to 5-hydroxytryptophan (5-HTP) catalyzed by tryptophan hydroxylase (TPH), which is the rate-limiting step of the pathway. Subsequently, 5-HTP decarboxylates to produce 5-hydroxytryptamine (serotonin, 5-HT) under the action of aromatic L-amino acid decarboxylase (AADC). Serotonin is a key neurotransmitter widely involved in mood regulation, sleep, appetite, and cognitive function. In addition, serotonin is further converted into melatonin in the pineal gland, which regulates circadian rhythms. This metabolic process is limited by tryptophan intake, TPH activity and metabolic status in the body, and has important effects on physical and mental health.
Microbial metabolic Pathways
The microbial metabolic pathway of tryptophan is dominated by the intestinal flora, mainly through two core enzyme systems: tryptophan enzyme catalyzes the deamination of tryptophan to generate indole, and aromatic amino acid decarboxylase converts it to tryptamine. The metabolites include indole derivatives (indole-propionic acid, indole-acetaldehyde) and tryptamines, among which indole substances enhance the expression of intestinal epithelial tight junction protein and maintain intestinal barrier function by activating the host aromatics receptor (AhR). Tryptamine mimics serotonin signaling and regulates the activity of the enteric nervous system. About 5-10% of dietary tryptophan is metabolized by intestinal flora, and its products circulate through the portal vein to the liver for secondary modifications (e.g., indole sulfation), and some circulate throughout the body to regulate distal organs. The host dynamically regulates the metabolic enzyme activity of the flora through the secretion of bile acids and antimicrobial peptides, forming a bidirectional interaction network. This pathway not only affects the flux allocation of tryptophan metabolism (which competes with the host kynuridine pathway as substrate), but also acts as a transboundary signaling molecule, integrating microbial metabolism with host physiological functions.
Tryptophan metabolic network
The tryptophan metabolic network is a highly dynamic and complex system that is regulated not only by nutritional status, oxygen supply, and signaling pathways such as HIF-1α and mTOR, but also by feedback from its own metabolites, such as the role of the NAD+/SIRT1 axis in energy metabolism and epigenetic regulation. At the same time, gut microbes are also deeply involved in metabolizing tryptophan to generate indole compounds and activate aromatic hydrocarbon receptor AhR signaling, thus affecting immune, neurological and metabolic homeostasis. These intertwined regulatory mechanisms ensure the rational distribution of tryptophan under different physiological conditions, so that it can not only meet the basic needs of protein synthesis, but also flexibly switch between the kynurenine pathway, serotonin synthesis and microbial metabolism. With a better understanding of these metabolic networks, we can not only more accurately analyze the role of tryptophan in health and disease, but also further explore how to optimize tryptophan metabolism through nutritional intervention, microbial regulation or gene editing to improve body health.
 Figure 2. The metabolic pathways of tryptophan.
Figure 2. The metabolic pathways of tryptophan.
Dynamic regulation mechanism of tryptophan
Regulation of nutrition and oxygen perception
The dynamic regulation of tryptophan is finely regulated by nutrient status and oxygen supply, in which HIF-1α (hypoxic-inducing-factory-1α) and mTOR (mammalian target of rapamycin) pathways play key roles. In hypoxic environment, HIF-1α is stably expressed, enhances the activity of indoleamino-2, 3-dioxygenase (IDO), accelerates the metabolism of tryptophan to kynurenine, and thus affects immune regulation and energy metabolism. In addition, the mTOR pathway acts as a cellular nutrient sensor to regulate tryptophan utilization according to amino acid supply levels. When tryptophan is abundant, mTOR signaling is enhanced, promoting protein synthesis and cell growth, while when nutrients are scarce, mTOR is inhibited, which may lead to more tryptophan being broken down into NAD+ or other metabolites to support cell survival and adaptation to environmental changes. This regulatory mechanism based on nutrient and oxygen status ensures the flexibility of tryptophan metabolism to adapt to different physiological needs.
Metabolite feedback regulation
Tryptophan metabolism is not only regulated by the external environment, but its metabolites also affect their own metabolic pathways through feedback mechanisms. Among them, NAD+ (nicotinamide adenine dinucleotide) is one of the important products of tryptophan kynuridine pathway, which is widely involved in energy metabolism and cell homeostasis maintenance. Fluctuations in NAD+ levels can directly affect the activity of SIRT1 (a NAD+ dependent deacetylase), and SIRT1 can regulate the expression of genes related to tryptophan metabolism through epigenetic regulation. For example, when NAD+ levels rise, SIRT1 inhibits mTOR signaling, promotes cellular adaptation to nutrient deficiency, enhances mitochondrial function, and enhances the body's tolerance to oxidative stress. In addition, SIRT1 affects the efficiency with which tryptophan is converted to serotonin, thereby indirectly regulating mood, sleep and neurological function. Feedback regulation of this metabolite-enzyme-signaling pathway ensures the metabolic homeostasis of tryptophan.
Gut microbial-host interaction
Gut microbiota plays a crucial role in tryptophan metabolism and its regulation, especially through the aromatic hydrocarbon receptor (AhR) pathway affecting host physiological functions. Certain gut microbes (such as Bacteroides and Firmicutes) are able to metabolize tryptophan to produce indole compounds such as indole propionic acid (IPA) and indole acetic acid (IAA), which act as ligands for AhR and activate the host's immune and metabolic regulatory network. Activation of AhR not only affects intestinal barrier integrity, but also regulates immune cell function, reduces inflammatory response, and to a certain extent affects neurotransmitter balance. In addition, AhR signaling pathway can also interact with metabolic regulatory axes such as mTOR and SIRT1 to further affect the metabolic flow of tryptophan. Through the close connection between intestinal microorganisms and host metabolic network, tryptophan metabolism can be dynamically regulated throughout the body, thus adapting to different physiological and environmental conditions.
Tryptophan is associated with human disease
Tryptophan metabolism is associated with neurological disorders
Disorders of tryptophan metabolism are closely related to a variety of neurological diseases. Abnormal activation of kynurenine pathway is the core mechanism: in depression, chronic inflammation induces IDO1 enzyme upregulation, resulting in tryptophan shunt to kynurenine, reducing 5-hydroxytryptophan (5-HT) synthesis, while excessive accumulation of quinolinic acid leads to glutaminergic excitotoxicity, forming a "monoamine-neurotoxicity" double-whammy model; In Alzheimer's disease patients, IDO1 activity is increased in the brain, kynurenine metabolite quinolinic acid induces neuronal calcium overload through the activation of NMDA receptors, and its concentration is positively correlated with the degree of tau phosphorylation. Parkinson's disease showed abnormal metabolism of tryptophan in intestinal flora, reduced microbial indole led to damage of intestinal barrier, and promoted intestinal pathological transmission of α-synuclein. In addition, insufficient 5-HT synthesis is associated with anxiety and obsessive-compulsive disorder, while dysregulation of kynuridine/AhR signaling is involved in autoimmune attack in multiple sclerosis. Peripheral tryptophan metabolites, such as the KYNA/QA ratio, have emerged as novel biomarkers for evaluating neuroinflammation, and regulatory strategies targeting IDO1 or AhR offer new directions for neurodegenerative disease therapy.
Tryptophan metabolism and cancer
Tryptophan metabolism is closely related to the occurrence and development of cancer, especially the role of Kynurenine pathway in the tumor microenvironment. Cancer cells often up-regulate indoleamine 2, 3-dioxygenase (IDO) or tryptophan 2, 3-dioxygenase (TDO) to accelerate tryptophan metabolism to kynurenine, inhibit T cell activity, and promote immune escape. In addition, kynuridine and its downstream metabolites can activate the aromatic hydrocarbon receptor (AhR) to further regulate the inflammatory response and the expression of tumor-related genes. At the same time, changes in the NAD+ metabolic pathway may also affect the energy supply and antioxidant capacity of cancer cells. Due to the central role of tryptophan metabolism in tumor immunosuppression, therapies targeting tryptophan metabolism such as IDO inhibitors have become an important direction of tumor immunotherapy.
Tryptophan and metabolic diseases
Tryptophan metabolism plays an important role in metabolic diseases such as obesity and insulin resistance. Obese individuals often exhibit overactivation of the kynurenine pathway, leading to accelerated degradation of tryptophan, and kynurenine and its metabolites can induce chronic inflammation by activating the aromatic hydrocarbon receptor (AhR), inhibiting the insulin signaling pathway, and promoting insulin resistance. In addition, indole compounds produced by intestinal microorganisms metabolizing tryptophan can affect intestinal barrier function and systemic inflammation, further exacerbating metabolic disorders. At the same time, the regulatory role of NAD+/SIRT1 axis in energy balance and fat metabolism is also affected by tryptophan metabolic status. Studies have shown that regulating tryptophan metabolism, such as inhibiting the kynurenine pathway or optimizing the gut microbiome, can improve insulin sensitivity and provide new ideas for the treatment of obesity and metabolic syndrome.
Conclusion
Tryptophan is an essential amino acid, and its metabolic network plays a key role in the regulation of health and disease. From kynuridine pathway to serotonin synthesis and intestinal microbial metabolism, the dynamic regulation of tryptophan not only affects immune, nervous and energy metabolism, but also is closely related to various pathological processes such as cancer and metabolic diseases. Signaling pathways such as HIF-1α, mTOR, NAD+/SIRT1 axis and AhR receptor jointly shape the metabolic direction of tryptophan, enabling it to adapt to the needs of different physiological states. A deeper understanding of this metabolic network could help shed light on disease mechanisms and provide potential targets for precision medicine. In the future, intervention strategies based on tryptophan metabolism, such as regulating gut microbiota, optimizing metabolic pathways or developing specific inhibitors, will likely become important directions for improving health and treating disease.
Reference
- Palego L, Betti L, Rossi A, Giannaccini G. (1972). Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans. J Amino Acids, 2016;2016:8952520. https://doi.org/10.1155/2016/8952520




