Cyanidin-3-O-glucoside (Chrysanthemin) Analysis Service
Creative Proteomics offers high-precision Cyanidin-3-O-glucoside (C3G) analysis using advanced LC-MS/MS and HPLC-UV platforms. Our services include ultra-sensitive quantification (LOD ≤ 0.1 ng/mL), identification of 20+ related metabolites such as cyanidin and protocatechuic acid, stability testing under pH, light, and temperature conditions, and comprehensive metabolic pathway analysis. We support quality control in food pigments, anthocyanin research in crop breeding, and antioxidant development in pharmaceuticals—delivering reliable, reproducible data for research and industrial R&D.
Submit Your Request Now
×- What We Provide
- Advantage
- Technology Platform
- Sample Requirements
- FAQ
- Publication
What is Cyanidin-3-O-glucoside?
Cyanidin-3-O-glucoside (C3G), also known as Chrysanthemin, is a prominent anthocyanin and the 3-O-glucoside derivative of cyanidin. It is widely distributed in nature, found in plants such as roselle (Hibiscus sabdariffa), purple corn (Zea mays), blackcurrant pomace, red raspberries, black rice, and red oranges. Structurally, it consists of a cyanidin aglycone linked to a glucose moiety, contributing to its antioxidant properties and role in plant pigmentation. C3G is also industrially significant; for example, purple corn color, rich in C3G, is approved in Japan as a food additive. Its biosynthesis involves enzymatic pathways, including glycosyltransferases like UGT79B1 in Arabidopsis thaliana, which further modifies C3G into derivatives such as cyanidin 3-O-xylosyl(1→2)glucoside.
Cyanidin-3-O-glucoside Analysis Service Offered by Creative Proteomics
- Quantitative Analysis of Cyanidin-3-O-glucoside: Precise quantification of C3G in various biological matrices including plant tissues, fruits, extracts, and processed food samples.
- Metabolite Profiling: Profiling the metabolic conversion of C3G to its metabolites, identifying key metabolites involved in C3G's biological functions.
- Identification of Related Anthocyanins: Identification and quantification of anthocyanins related to C3G, allowing for a comprehensive study of the flavonoid content in a sample.
- Pathway Analysis: Detailed analysis of the metabolic pathways involving C3G, using both qualitative and quantitative data to map out interactions and transformations within metabolic networks.
- Stability and Degradation Studies: Investigation of the stability of C3G under various environmental and storage conditions, useful for food scientists and pharmaceutical developers.
Detected Cyanidin-3-O-glucoside-Related Metabolites
| Compound Name | Type | Metabolic Pathway |
|---|---|---|
| Cyanidin-3-O-glucoside (C3G) | Parent compound | Anthocyanin biosynthesis |
| Cyanidin | Aglycone | Glycosylation/de-glycosylation |
| Cyanidin-3-O-rutinoside | Glycosylated derivative | Anthocyanin biosynthesis |
| Cyanidin-3,5-di-O-glucoside | Diglycosylated anthocyanin | Flavonoid modification |
| Cyanidin-3-O-galactoside | Sugar isomer of C3G | Sugar conjugation pathway |
| Cyanidin-3-O-arabinoside | Sugar conjugate | Sugar conjugation pathway |
| Protocatechuic acid | Degradation product | Anthocyanin degradation |
| Phloroglucinaldehyde | Degradation product | Anthocyanin degradation |
| Peonidin-3-O-glucoside | Methylated anthocyanin | Methylation of C3G |
| Malvidin-3-O-glucoside | Related anthocyanin | Anthocyanin extension pathway |
| Delphinidin-3-O-glucoside | Related anthocyanin | Flavonoid biosynthesis |
| Ferulic acid | Downstream metabolite | Phenylpropanoid pathway |
| Quercetin | Related flavonol | Flavonoid metabolism |
| Kaempferol | Related flavonol | Flavonoid backbone pathway |
Advantages of Cyanidin-3-O-glucoside Assay
- Ultra-High Sensitivity & Accuracy: Utilizing LC-MS/MS technology, our service achieves detection limits as low as 0.1 ng/mL for Cyanidin-3-O-glucoside, ensuring precise quantification even in trace-level samples.
- Broad Analytical Coverage: Our platform detects over 20 related metabolites and derivatives of Cyanidin-3-O-glucoside, enabling comprehensive profiling across flavonoid biosynthesis and anthocyanin metabolic pathways.
- Fast Turnaround Time: With optimized workflows and automated sample handling, we deliver results in as fast as 7 business days, even for batches exceeding 96 samples.
- High Reproducibility: All analyses are performed under strict QC parameters with technical repeatability (RSD%) maintained under 5%, ensuring consistent, publication-grade data.
- Flexible Sample Compatibility: Compatible with diverse matrices including fresh plant tissues, fruit juices, food extracts, and purified standards—no need for extensive preprocessing.
Technology Platform for Cyanidin-3-O-glucoside Analysis Service
Thermo Fisher Scientific Q Exactive Orbitrap Mass Spectrometer: Ensures highly accurate mass measurement, providing precise identification of C3G and its metabolites.
AB Sciex Triple Quad 6500+: Used for targeted quantification of C3G and its metabolites, offering highly sensitive detection capabilities.
Agilent 1290 Infinity II HPLC System: Provides exceptional chromatographic separation to isolate C3G and related compounds from complex samples.

Q Exactive™ Plus Hybrid Quadrupole-Orbitrap™ Mass Spectrometer (Figure from Thermo)

SCIEX Triple Quad™ 6500+ (Figure from Sciex)

Agilent 1260 Infinity II HPLC (Figure from Agilent)
Sample Requirements for Cyanidin-3-O-glucoside Analysis Service
| Sample Type | Minimum Amount Required | Storage Condition | Additional Notes |
|---|---|---|---|
| Fresh Plant Tissue | ≥ 1 g | -80°C or liquid nitrogen | Avoid repeated freeze-thaw cycles |
| Freeze-Dried Tissue | ≥ 200 mg | Room temp (dry & sealed) | Store in moisture-free environment |
| Fruit or Vegetable Juice | ≥ 10 mL | -20°C | Filter to remove pulp before shipping |
| Extract (Methanol/EtOH) | ≥ 500 µL | -20°C | Use HPLC-grade solvents only |
| Fermented Product | ≥ 5 mL | -20°C | Centrifuge to remove solids if present |
| Standard Compound | ≥ 0.1 mg | -20°C (light-protected) | Dissolve in appropriate solvent |
Applications of Cyanidin-3-O-glucoside Assay Service
Agricultural Research
Studying the biosynthesis of anthocyanins in plants and crops to improve pigment production.
Food Science
Quantification of C3G in fruits, vegetables, and processed food products.
Environmental Science
Studying the impact of environmental factors on C3G content in plants and fruits.
Pharmaceutical Research
Analyzing the potential antioxidant and anti-inflammatory properties of C3G in medicinal products.
Demo
FAQ of Cyanidin-3-O-glucoside Analysis Service
How should samples be prepared for C3G analysis?
Samples should be homogenized and extracted using polar solvents like methanol, ethanol, or acidified aqueous solutions (e.g., 1% HCl in methanol). For plant tissues, freeze-drying and grinding before solvent extraction improve yield. Ensure samples are stored at 2–8°C and protected from light to prevent degradation.
What is the typical detection limit for C3G in HPLC analysis?
The detection limit varies by instrument, but HPLC with UV-Vis detection at 520 nm typically achieves a limit of quantification (LOQ) around 0.1 µg/mL. LC-MS/MS can detect concentrations as low as 25 µg/L in complex matrices.
How does pH affect C3G stability during analysis?
C3G is sensitive to pH changes. Acidic conditions (pH < 3) stabilize its structure, while neutral or alkaline conditions accelerate degradation. Always use acidified solvents (e.g., 0.2% phosphoric acid) in mobile phases for HPLC to maintain stability.
What are the key applications of C3G analysis?
C3G analysis is critical for studying its antioxidant, anti-inflammatory, and anti-cancer properties. It is also used in food science to evaluate pigment stability in products like black rice, purple sweet potatoes, and berries.
Can C3G be analyzed alongside other anthocyanins?
Yes, gradient elution in HPLC or LC-MS/MS allows simultaneous quantification of C3G and other anthocyanins (e.g., pelargonidin, delphinidin derivatives). A C18 column with a water-acetonitrile-phosphoric acid mobile phase is commonly used for separation.
What are the common challenges in C3G analysis?
Key challenges include light-induced degradation, thermal instability during extraction, and matrix interference in plant samples. Using amber vials, low-temperature processing, and solid-phase extraction (SPE) cleanup can mitigate these issues.
How is C3G quantified in biological samples (e.g., plasma or tissues)?
Biological samples require protein precipitation (e.g., using methanol or acetonitrile) followed by SPE or liquid-liquid extraction. LC-MS/MS with isotope-labeled internal standards (e.g., cyanidin-3-glucoside-d3) enhances accuracy.
What quality control measures are recommended?
Include blank samples, spike-recovery tests, and reference standards in each batch. Store reference standards at -80°C and verify purity (>98%) via HPLC before use.
How can C3G degradation products be identified?
Degradation products (e.g., cyanidin or protocatechuic acid) are identified using LC-MS/MS with fragmentation patterns. Stability studies under varying temperatures and light exposure help characterize degradation pathways.
Learn about other Q&A.
Cyanidin-3-O-glucoside Analysis Service Case Study
Publications
Here are some publications in Metabolomics research from our clients:

- Thermotolerance capabilities, blood metabolomics, and mammary gland hemodynamics and transcriptomic profiles of slick-haired Holstein cattle during mid lactation in Puerto Rico. 2024. https://doi.org/10.3168/jds.2023-23878
- Overexpression of maize ZmLOX6 in Arabidopsis thaliana enhances damage-induced pentyl leaf volatile emissions that affect plant growth and interaction with aphids. 2023. https://doi.org/10.1093/jxb/erac522
- High Levels of Oxidative Stress Early after HSCT Are Associated with Later Adverse Outcomes. 2024. https://doi.org/10.1016/j.jtct.2023.12.096
- Macrophage-associated lipin-1 promotes β-oxidation in response to proresolving stimuli. 2020. https://doi.org/10.4049/immunohorizons.2000047
- Comparative metabolite profiling of salt sensitive Oryza sativa and the halophytic wild rice Oryza coarctata under salt stress. 2024. https://doi.org/10.1002/pei3.10155
Reference
- Wongsa, Prinya. "Phenolic compounds and potential health benefits of pigmented rice." Recent advances in rice research. IntechOpen, 2020. https://doi.org/10.5772/intechopen.93876

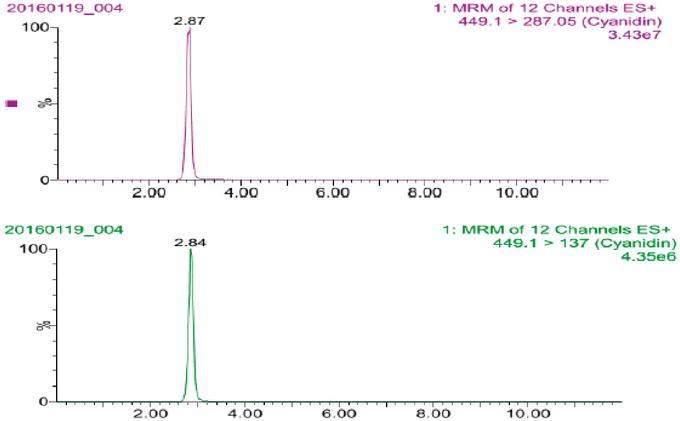
![(a) MS/MS spectrum of cyanidin 3-O-glucoside. (b) MS/MS spectrum of pelargonidin-3-O-glucoside. (c) MS/MS spectrum of peonidin 3-O-glucoside [13].](/upload/image/pic-cyanidin-3-o-glucoside-chrysanthemin-analysis-service-5.jpg)







