Anthocyanins Profiling and Quantification Service
Anthocyanins are vibrant but chemically complex—prone to degradation, sensitive to processing, and challenging to quantify precisely. At Creative Proteomics, we provide advanced UHPLC–MS/MS-based anthocyanin profiling solutions to help you resolve isomers, verify botanical origin, and track formulation impacts. Whether you're in R&D, quality assurance, or regulatory, our methods offer decision-grade insights to move your product forward with confidence.
Why Choose Our Anthocyanin Analysis Services?
- Targeted quantification of key anthocyanins using isotope-aided LC–MS/MS
- Isomer resolution via high-resolution MS/MS and tailored MRM transitions
- Authenticity fingerprinting to verify varietal or botanical identity
- Process impact studies covering pH, heat, light, and storage conditions
- Color stability & co-pigment assessment for formulation guidance
Submit Your Request Now
×
- What We Provide
- Advantages
- Technology Platform
- Sample Requirements
- Demo
- FAQs
What Are Anthocyanins?
Anthocyanins are water-soluble flavonoid pigments that give plants red, purple, or blue hues. They typically exist as glycosides of anthocyanidins with diverse sugars and acyl groups. These structural features influence color stability, antioxidant behavior, and bioavailability in complex matrices.
Precise profiling supports authenticity checks, cultivar screening, and process optimization in food and botanicals. Researchers also track anthocyanin dynamics to study stress responses and metabolic pathways. For product developers, reliable data informs formulation choices and shelf-life strategies.
What Problems Do We Help You Solve?
- Isomer resolution: Differentiate close structural isomers and acylated forms that co-elute in basic methods.
- Matrix interference: Reduce background from complex food, plant, and fermentation matrices.
- Stability risk: Prevent degradation from pH, temperature, light, and metal ions.
- Quantification gaps: Convert semi-quantitative screens into calibrated, reportable concentrations.
Targeted Anthocyanin Profiling & Quantification Solutions
![]()
Targeted Quantification of Core Anthocyanins
Accurate quantification of major anthocyanin classes using isotope-labeled standards:
- Cyanidin, delphinidin, malvidin, peonidin, pelargonidin, petunidin
- Isotope-dilution calibration for enhanced quant reliability
- Applicable to juices, berries, cereals, red wines, and botanical extracts
![]()
Analysis of Glycosylated & Acylated Derivatives
Comprehensive coverage of anthocyanin conjugates:
- 3-O-glucosides, diglycosides (e.g., rutinosides, sambubiosides)
- Acylated derivatives with p-coumaroyl, caffeoyl, feruloyl, or sinapoyl groups
- Essential for full profiling of plant-derived and functional products
![]()
High-Resolution MS/MS for Structure Confirmation
Confident identification of near-isomers and complex structures via HRAM:
- Discrimination of glycosylation and acylation patterns
- Resolves co-eluting or ambiguous chromatographic peaks
- Critical for accurate structural elucidation in complex matrices
![]()
Botanical Origin & Authenticity Testing
Fingerprint anthocyanin profiles to verify identity and origin:
- Support for varietal, species, and geographical claims
- Detection of adulteration or substitution in raw materials and finished goods
- Valuable for regulatory submissions and brand assurance
![]()
Processing & Formulation Impact Studies
Evaluate anthocyanin stability and transformation during product development:
- Thermal treatment, pH adjustment, blending, fermentation
- Shelf-life studies under light, heat, and oxidative conditions
![]()
Color Stability & Co-pigmentation Effect Assessment
Connect chemical profiles with color performance:
- Impact of co-pigments (e.g., flavonols) and metal ions on anthocyanin color
- Useful for natural colorant stability evaluation and enhancement strategies
Detectable Anthocyanins: Comprehensive Panel
| Group / Class | Representative analytes (non-exhaustive) | Notes |
|---|---|---|
| Anthocyanidin aglycones | Cyanidin; Delphinidin; Malvidin; Pelargonidin; Peonidin; Petunidin | Free (non-glycosylated) forms |
| 3-O-monoglycosides (for each aglycone) | 3-O-glucoside; 3-O-galactoside; 3-O-arabinoside; 3-O-rhamnoside; 3-O-xyloside | Core plant and food glycosides |
| Di-glycosides / mixed linkages | 3,5-di-O-glucoside; 3-O-rutinoside; 3-O-sambubioside; 3-O-sophoroside; 3-O-neohesperidoside; 3-O-gentiobioside; 3-O-laminaribioside | Frequent in berries, grapes, botanicals |
| Acylated mono-conjugates | Acetyl-glc; Malonyl-glc; p-hydroxybenzoyl-glc; p-coumaroyl-glc; Caffeoyl-glc; Feruloyl-glc; Sinapoyl-glc | Acyl position per natural occurrence (e.g., 6'' on glucose) |
| Acylated di/tri-conjugates | di-p-coumaroyl; p-coumaroyl+caffeoyl; p-coumaroyl+feruloyl; caffeoyl+feruloyl; feruloyl+sinapoyl; tri-acyl variants | Observed in highly pigmented cultivars |
| Cyanidin series | Cyanidin-3-O-glucoside (kuromanin); -galactoside; -arabinoside; -rutinoside; 3,5-di-O-glc; 6''-p-coumaroyl-glc; acetyl/malonyl/caffeoyl/feruloyl derivatives | Broad distribution across fruits and grains |
| Delphinidin series | Delphinidin-3-O-glc (myrtillin); -galactoside; -arabinoside; -rutinoside; 3,5-di-O-glc; p-coumaroyl/caffeoyl/feruloyl/sinapoyl acylates | Common in dark berries and flowers |
| Malvidin series | Malvidin-3-O-glc (oenin); -galactoside; -arabinoside; -rutinoside; 3,5-di-O-glc; 6''-acetyl-, 6''-p-coumaroyl-, 6''-caffeoyl-glc; di-acyl forms | Characteristic in Vitis spp. (grapes, wines) |
| Pelargonidin series | Pelargonidin-3-O-glc (callistephin); -rutinoside; -galactoside; acetyl/p-coumaroyl/malonyl derivatives | Typical of strawberries and red petals |
| Peonidin series | Peonidin-3-O-glc (peonin); -rutinoside; -galactoside; 3,5-di-O-glc; p-coumaroyl/acetyl/malonyl derivatives | Found in grapes and red plant tissues |
| Petunidin series | Petunidin-3-O-glc; -rutinoside; -galactoside; caffeoyl/feruloyl/p-coumaroyl acylates; 3,5-di-O-glc | Reported in berries and grapes |
| Processing-derived pigments (on request) | Pyranoanthocyanins (e.g., vitisin A/B); ethyl-linked derivatives; vinylphenol-pyranoanthocyanins; C-glycosyl anthocyanins; anthocyanin–tannin adducts (class profiling) | Matrix-dependent detectability |
| Typical matrices | Berries; grapes and wines; purple corn; colored rice and wheat; red cabbage; eggplant peel; flowers; teas and botanicals; juices; fermented beverages; nutraceutical extracts | Selection informs panel design |
Why Choose Creative Proteomics for Anthocyanin Analysis?
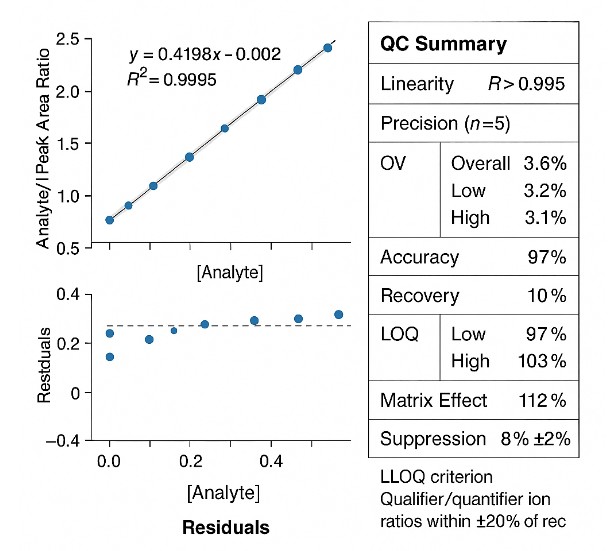
- Decision-grade quantification: Targeted LC–MS/MS with isotope aid; calibrations designed for R2 ≥ 0.995 and CV ≈ 10–15% when matrices allow.
- Isomer clarity: UHPLC selectivity plus tailored MRM and confirmatory MS/MS to resolve near-isomers and acylates.
- Stability-aware handling: Acidified, low-light, chilled workflows with optional chelators to protect labile pigments.
- Matrix-adaptive methods: Gradients and cleanup tuned for berries, wines, grains, botanicals, juices, and ferments.
- Transparent ID rules: Tiered criteria: precursor/fragment match, retention logic, acylation pattern, optional HRAM.
- End-to-end QC: Blanks, checks, and matrix QCs with calibration plots and flagged results in reports.
Anthocyanin Analysis Workflow: Step-by-Step Process
- Scoping & panel design
Define matrices, target anthocyanins, reporting units, and decision criteria. - Sample intake & handling
Verify packaging; store cold and protected from light to limit degradation. - Extraction & cleanup
Use acidified, light-shielded protocols with internal standards to improve recovery. - UHPLC separation
Select C18 or phenyl-hexyl phases; tune gradients for glycosides and acylates. - LC–MS/MS acquisition
Acquire targeted MRM; trigger confirmatory MS/MS or HRAM when identities are uncertain. - Processing & QC review
Calibrate, check matrix effects, verify IDs, and compile traceable QC artifacts. - Reporting & interpretation
Deliver concentration tables, annotated chromatograms, MS/MS evidence, and concise next-step notes.

Anthocyanin Analysis Instrumentation: Core Models & Key Parameters
UHPLC (primary): Thermo Vanquish UHPLC; Agilent 1290 Infinity II.
- Columns: C18 or phenyl-hexyl (≈2.1 × 100 mm, 1.7–2.6 µm) with guard.
- Typical LC setup: Column 30–40 °C; injection 1–5 µL; water/0.1% formic acid–acetonitrile/0.1% formic acid gradients.
Targeted LC–MS/MS (quant): SCIEX 6500+ and Thermo TSQ Altis.
- Mode & method: ESI (+), scheduled MRM; 2–4 transitions per analyte; matrix-matched or isotope-aided calibration where available.
High-resolution confirmation: Thermo Orbitrap Exploris 480.
- Use & setup: Full-scan HRAM with data-dependent MS/MS; lock-mass or external calibration for mass accuracy.
Optical support: Integrated DAD/UV-Vis (peak purity/color tracking around 520–540 nm).

1260 Infinity II HPLC (Figure from Agilent)

Vanquish UHPLC (Figure from Thermo)

Triple Quad™ 6500+ (Figure from Sciex)

Orbitrap Exploris 480 (Figure from Thermo)
Sample Requirements for Anthocyanin Analysis Service
| Matrix type | Typical examples | Preferred container | Light/temperature protection | Suggested amount* | Notes |
|---|---|---|---|---|---|
| Fresh/frozen plant tissue | Berry flesh/skin, grape skins, red cabbage, eggplant peel, leaf tissue | Amber screw-cap tube or foil-wrapped cryovial | Keep cold; minimize light; avoid freeze–thaw | 0.5–2 g | Record species, part, harvest/pretreatment; avoid metal contact. |
| Dried plant powder | Berry/grape skin powder, botanical extracts (dry), colored grain flour | Amber glass vial with PTFE-lined cap | Dry, cool, light-protected | 50–200 mg | Provide milling method; avoid anti-caking agents and unknown stabilizers. |
| Juices & aqueous beverages | Berry/grape juices, kombuchas, plant infusions | Amber polypropylene tube or amber glass | Cold chain; protect from light | 3–10 mL | Note pH and any preservatives; avoid metal ions that catalyze loss. |
| Alcoholic beverages | Red wine, berry wines, tinctures | Amber glass vial with PTFE cap | Cool, light-protected | 3–10 mL | Provide ethanol %; list fining/filtration steps if known. |
| Liquid botanical extracts | Hydroalcoholic or glycerol extracts | Amber glass vial | Cold chain; shield from light | 2–5 mL | Include solvent system and dilution factor. |
| Semi-solids & concentrates | Jams, purees, syrups, concentrates | Wide-mouth amber jar/tube | Cold; minimize headspace; light-protected | 2–5 g | Indicate added sugars/acids; note processing steps (heating). |
| Finished products (RUO) | Capsules, tablets, gummies, drink mixes | Original packaging + secondary amber container | Cool, dry; away from light | 5–10 units or 2–5 g composite | Provide label actives; send composite sample to reduce unit variability. |
| Fermentation samples | Musts, ferments, culture broths | Amber tube or bottle, gas-permeation minimized | Cold; protected from light | 3–10 mL | Note fermentation stage and any pH adjustments. |
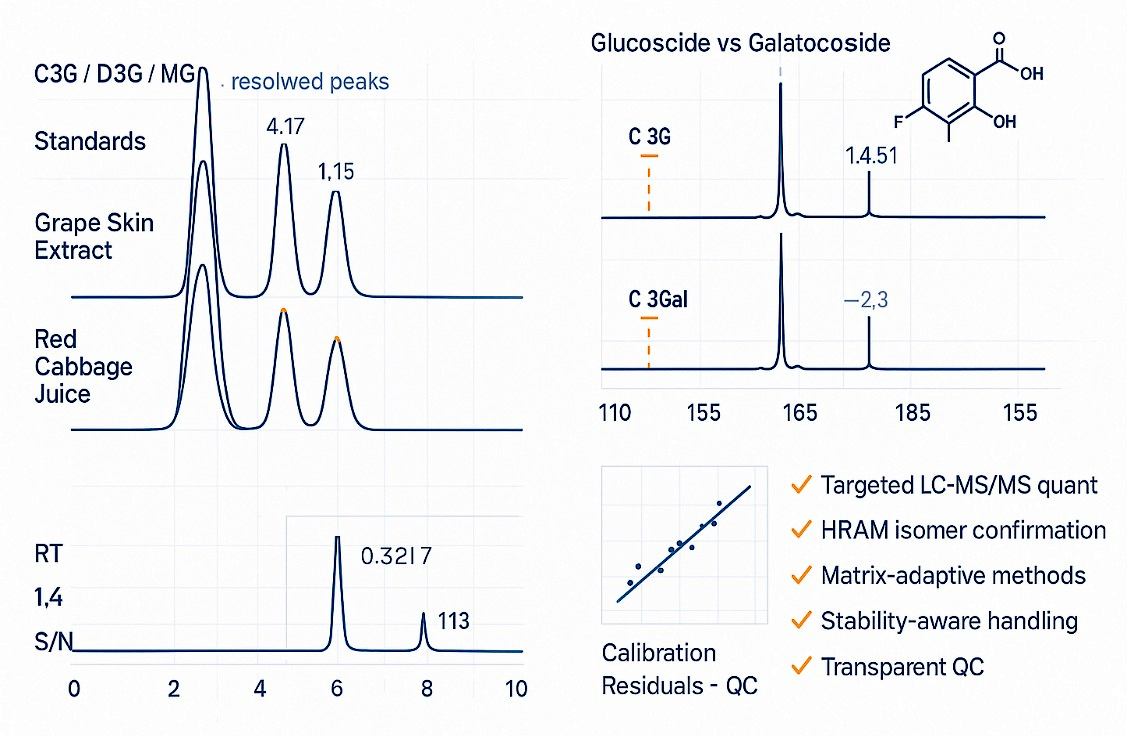
Demo Results
FAQ of Anthocyanin Analysis Service
What's the difference between anthocyanins and anthocyanidins?
Anthocyanidins are the aglycone pigments; anthocyanins are their sugar-bound (and often acylated) glycosides—both drive red–blue coloration in plants.
Which analytical methods are best for anthocyanin measurement?
HPLC-DAD is widely used for routine profiling, while LC–MS/MS provides sensitive, selective quantification and structural clues; both are commonly paired in validated workflows.
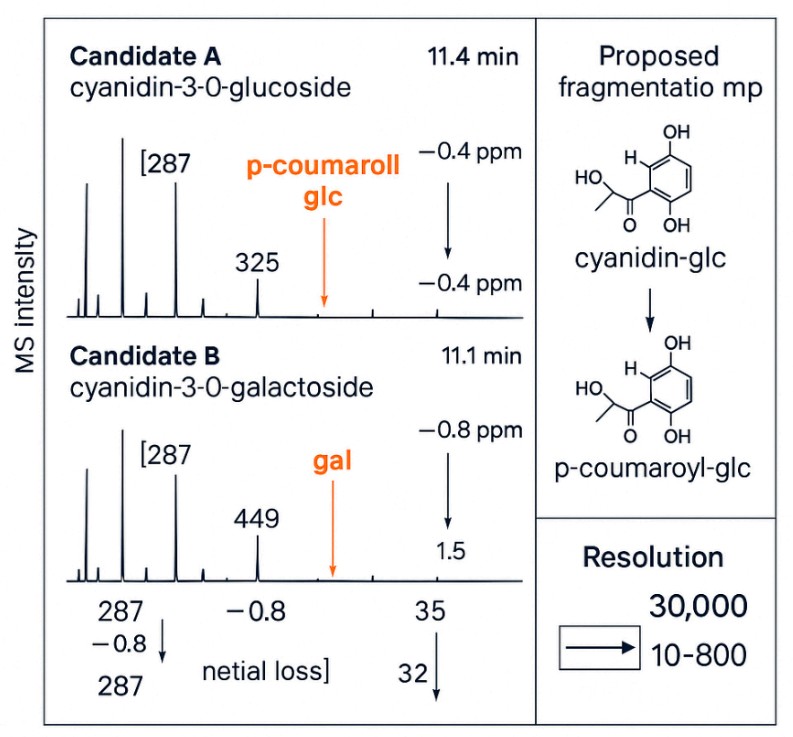
When do I need high-resolution MS/MS (HRAM)?
Use HRAM to confirm near-isomers (e.g., glucoside vs galactoside) and to map acylation patterns or ambiguous co-eluting peaks—improving annotation confidence.
How does pH affect anthocyanin color and stability?
Anthocyanins are most stable and intensely colored in strong acid; higher pH shifts forms and can reduce color and stability, so matrices and buffers matter.
Do metal ions and light/heat really change results?
Yes—metal chelation, light, oxygen, and temperature accelerate degradation or hue shifts; method design and handling must control these factors.
What is the AOAC pH differential method and when is it useful?
It's a spectrophotometric approach that reports total monomeric anthocyanins based on pH-dependent absorbance (pH 1.0 vs 4.5); ideal for rapid screening and labeling checks.
What is copigmentation and why should I care?
Noncovalent complexes with phenolic co-pigments can stabilize color (hyperchromic/bathochromic effects) and improve shelf-appearance in products.
LC–MS/MS vs HPLC-DAD: how do I choose?
Pick HPLC-DAD for economical routine panels and total trends; choose LC–MS/MS when you need isomer discrimination, lower detection limits, and robust quantification in complex matrices.
Can you quantify acylated and diglycoside forms without neat standards?
Yes—labs often use surrogate standards plus MS/MS criteria, but reports should state assumptions and qualifier/quantifier rules clearly.
Which sample factors most often compromise results?
Uncontrolled pH, light/heat, reactive metals, and repeated freeze–thaw cycles drive losses; optimized acidified, low-light handling mitigates these risks.
How do "total anthocyanins" results compare with LC–MS/MS totals?
The pH differential method measures monomeric forms on a reference basis (often C3G) and excludes polymeric pigments; targeted LC–MS/MS sums named species and derivatives—values are not directly interchangeable.
Where are anthocyanins most commonly found?
They're abundant in berries, grapes/wine, purple corn, colored grains, red cabbage, and many botanicals, typically as glycosides of cyanidin, delphinidin, malvidin, pelargonidin, peonidin, and petunidin.
Learn about other Q&A about proteomics technology.
Publications
Here are some of the metabolomics-related papers published by our clients:

- Plant Growth Promotion, Phytohormone Production and Genomics of the Rhizosphere-Associated Microalga, Micractinium rhizosphaerae sp. 2023
- Overexpression of maize ZmLOX6 in Arabidopsis thaliana enhances damage-induced pentyl leaf volatile emissions that affect plant growth and interaction with aphids. 2023
- Characterization of CYCLOPHILLIN38 shows that a photosynthesis-derived systemic signal controls lateral root emergence. 2021
- Summative and ultimate analysis of live leaves from southern US forest plants for use in fire modeling. 2020
- Disruption of CYCLOPHILIN 38 function reveals a photosynthesis-dependent systemic signal controlling lateral root emergence. 2020