Straight Chain Fatty Acids Analysis Service
Creative Proteomics delivers high-sensitivity straight chain fatty acids analysis using advanced GC-MS and LC-MS platforms, providing accurate quantification across diverse sample types. Our service supports researchers in lipid metabolism, nutrition, microbiome, and metabolic pathway studies by offering reliable, reproducible data to uncover biochemical insights and drive decision-making in complex biological systems.
Submit Your Request Now
×- What We Provide
- Advantage
- Workflow
- Technology Platforms
- Sample Requirements
- FAQ
- Publication
What are Straight Chain Fatty Acids?
Straight chain fatty acids are the basic building block in the universe of lipids. They are composed of a carboxylic acid with a long and unbranched chain of carbon atoms. The number of carbons in the fatty acid varies among species. In higher organisms like mammals, fatty acids always consist of an even number of carbons, however in lower organisms like bacteria, odd number of carbon also exists. Most naturally occurring fatty acids have 4 to 26 carbons, but longer fatty acids of up to 35 carbons are also described in literature.
According to the number of carbons, straight chain fatty acids can be classified into short chain fatty acids (straight chain fatty acid, C2–C7), medium chain fatty acids (MCFA, C8–C13), long chain fatty acids (LCFA, C14–C19), and very long chain fatty acids (VLCFA, C20+). Straight chain fatty acids are either saturated or unsaturated with one or more double bonds.
 Figure 1. Straight chain fatty acids: hexadecanoic acid
Figure 1. Straight chain fatty acids: hexadecanoic acid
In cells, straight chain fatty acids play important biological roles. They have a variety functions, including being incorporated into membrane for membrane proliferation, energy storage, generation of signaling molecules, or oxidized to carbon dioxide as energy source. The levels of straight chain fatty acids are sensitive to metabolic perturbations, therefore, they can be served as good markers for many health conditions and diseases. So far, the profiling of straight chain fatty acid has lead to the discovery of many disease markers, including insulin resistance, type 2 diabetes, obesity, cardiovascular diseases, cancers, and many other diseases.
Creative Proteomics offers a comprehensive profiling of straight chain fatty acids that satisfy a variety of purposes. GC/MS platform enables more sensitive and selective assay of fatty acid measurement. GC/MS techniques serves as a powerful analytical tool for identification and quantification of fatty acids.
Straight Chain Fatty Acids Analysis Service Offered by Creative Proteomics
- Quantitative Analysis of Straight Chain Fatty Acids: Accurate measurement of straight chain fatty acids in plasma, serum, urine, feces, tissues, cell cultures, and fermentation media using advanced GC-MS/MS and LC-MS platforms.
- Comprehensive Fatty Acid Profiling: Simultaneous analysis of straight chain, branched-chain, and unsaturated fatty acids for complete lipidomic profiling.
- Acyl-CoA Derivatives Detection: Targeted detection of key intermediates such as acetyl-CoA, propionyl-CoA, butyryl-CoA, and other short- to medium-chain acyl-CoAs.
- Quantification of Related Metabolites: Measurement of downstream compounds including β-hydroxybutyrate, succinyl-CoA, and methylmalonyl-CoA to support pathway-level analysis.
- Custom Metabolic Pathway Panels: Focused analysis of specific pathways such as butanoate metabolism, propanoate metabolism, fatty acid β-oxidation, and ketogenesis.
- Stable Isotope Labeling (Optional): ¹³C- or ²H-labeled fatty acid tracing for metabolic flux analysis in cells or animal models.
- Optimized Sample Preparation: Customized extraction protocols for complex matrices such as liver, feces, and microbial cultures, ensuring high recovery and reproducibility.
List of Detected Straight Chain Fatty Acids and Related Metabolites
| Straight Chain Fatty Acid | Related Metabolites | Detectable Compounds in Associated Pathways | Major Metabolic Pathways |
|---|---|---|---|
| Acetic acid (C2:0) | Acetyl-CoA, Acetate esters | Citrate, Oxaloacetate, Pyruvate, Ethanol | Glycolysis, TCA cycle, Acetate metabolism |
| Propionic acid (C3:0) | Propionyl-CoA, Succinyl-CoA | Methylmalonyl-CoA, Odd-chain fatty acids | Propanoate metabolism, Gluconeogenesis |
| Butyric acid (C4:0) | Butyryl-CoA, GABA | β-Hydroxybutyrate, Crotonyl-CoA | Butanoate metabolism, Ketogenesis |
| Valeric acid (C5:0) | Valeryl-CoA | Isovaleryl-CoA, 3-Methylcrotonyl-CoA | Amino acid degradation (Leu, Ile) |
| Caproic acid (C6:0) | Hexanoyl-CoA | Acetoacetate, Acyl-carnitines | Fatty acid β-oxidation, Lipid metabolism |
| Enanthic acid (C7:0) | Heptanoyl-CoA | 3-Hydroxyheptanoate, Acyl-ACP | Peroxisomal oxidation, Chain elongation |
| Caprylic acid (C8:0) | Octanoyl-CoA, Ketone bodies | β-Hydroxybutyrate, Acetoacetate | Mitochondrial β-oxidation, Ketogenesis |
| Pelargonic acid (C9:0) | Nonanoyl-CoA | Dicarboxylic acids, ω-Oxidation products | Peroxisomal metabolism, Detoxification |
| Capric acid (C10:0) | Decanoyl-CoA | Medium-chain acyl-carnitines | Medium-chain fatty acid metabolism |
| Undecanoic acid (C11:0) | Undecanoyl-CoA | ω-Hydroxy acids | Elongation & ω-oxidation |
| Lauric acid (C12:0) | Lauroyl-CoA | Dodecanedioic acid | Lipid biosynthesis, β-oxidation |
| Tridecanoic acid (C13:0) | Tridecanoyl-CoA | Odd-chain carnitine esters | Branched and odd-chain fatty acid pathways |
| Myristic acid (C14:0) | Myristoyl-CoA | Malonyl-CoA, Palmitic acid | Lipogenesis, Fatty acid elongation |
| Pentadecanoic acid (C15:0) | Pentadecanoyl-CoA | Propionyl-CoA, Succinyl-CoA | Odd-chain fatty acid metabolism |
| Palmitic acid (C16:0) | Palmitoyl-CoA | Stearoyl-CoA, Acyl-ACP | Lipogenesis, Elongation, β-oxidation |
| Heptadecanoic acid (C17:0) | Heptadecanoyl-CoA | Methylmalonyl-CoA | Mitochondrial oxidation, Propanoate metabolism |
| Stearic acid (C18:0) | Stearoyl-CoA | Oleoyl-CoA, Arachidoyl-CoA | Lipid storage, Saturated fat metabolism |
Advantages of Straight Chain Fatty Acids Assay
- Detection sensitivity reaches as low as 5 ng/mL, enabling accurate measurement of trace-level straight chain fatty acids in complex biological samples such as feces, plasma, and tissue extracts.
- Intra-assay precision consistently achieves a coefficient of variation (CV) below 8%, ensuring exceptional reproducibility within each analytical batch.
- Inter-assay precision remains below 12%, providing reliable performance across different runs and extended timeframes.
- Quantification is linear across a dynamic range of 10⁴ to 10⁸ ng/mL, allowing simultaneous analysis of both low-abundance and high-abundance fatty acids without dilution.
- Up to 96 samples can be processed in a single batch, supporting high-throughput project timelines and large-scale study designs.
- Recovery rates range from 85% to 110%, optimized for various sample matrices including serum, urine, feces, tissue homogenates, and microbial culture media.
- Signal-to-noise ratios exceed 300:1 when using our GC-MS/MS platform, delivering high-resolution, low-background quantification even in complex matrices.
- Sample stability is maintained for over 72 hours at −80°C, with less than 5% degradation, ensuring consistent results during storage and transport.
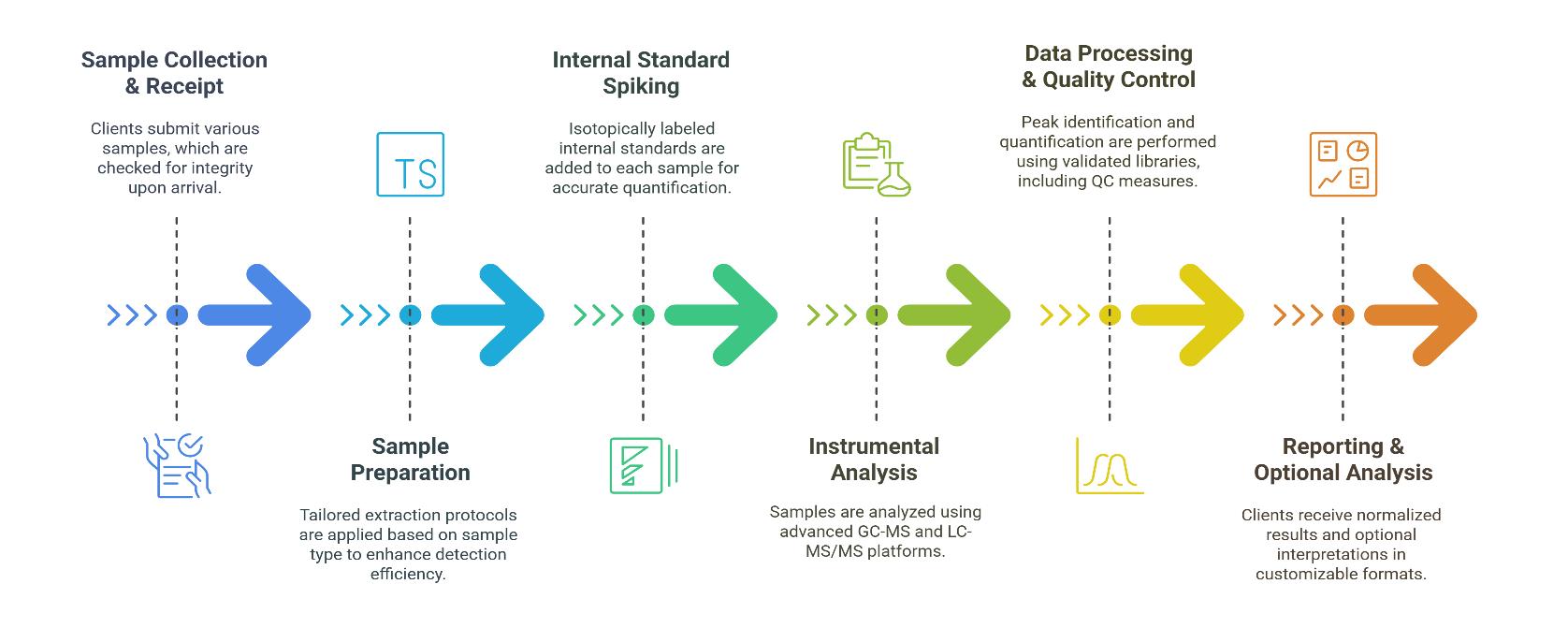
Workflow for Straight Chain Fatty Acids Analysis Service
Technology Platform for Straight Chain Fatty Acids Analysis Service
Gas Chromatography-Mass Spectrometry (GC-MS)
- Agilent 7890B Gas Chromatograph + 5977A Mass Selective Detector
Ideal for volatile fatty acids (C2–C6); provides excellent separation and quantification via electron ionization (EI) mode.
→ Typical LOD: 5–10 ng/mL
→ Used with fatty acid methyl ester (FAME) derivatization
Liquid Chromatography-Mass Spectrometry (LC-MS/MS)
- Thermo Scientific Q Exactive™ Plus Orbitrap MS + UltiMate 3000 UHPLC
High-resolution accurate mass (HRAM) system used for medium-to-long chain straight chain fatty acids and polar metabolites.
→ Resolving power: up to 140,000 FWHM
→ Mass accuracy:<3 ppm
→ Suitable for untargeted metabolomics panels - AB Sciex Triple Quad™ 6500+ LC-MS/MS
Targeted quantification with enhanced sensitivity using scheduled MRM.
→ Ideal for high-throughput, absolute quantification workflows
→ LLOQ as low as 1–2 ng/mL for selected analytes

Agilent 7890B-5977A (Figure from Agilent)

SCIEX Triple Quad™ 6500+ (Figure from Sciex)

Thermo Fisher Q Exactive (Figure from Thermo Fisher)
Sample Requirements for Straight Chain Fatty Acids Analysis Service
| Sample Type | Minimum Amount Required | Storage Condition | Notes |
|---|---|---|---|
| Plasma / Serum | ≥ 100 µL | -80°C | Avoid hemolysis; use EDTA or heparin tubes |
| Tissue (e.g., brain, liver) | ≥ 100 mg | Snap-frozen, -80°C | Remove blood contamination if possible |
| Cultured Cells | ≥ 5 × 10⁶ cells | Cell pellet, -80°C | Wash with PBS before freezing |
| CSF (Cerebrospinal Fluid) | ≥ 100 µL | -80°C | Preferably collected in polypropylene tubes |
| Urine | ≥ 500 µL | -80°C | Collect midstream sample; centrifuge before freezing |
| Isolated Lipid Extracts | ≥ 50 µg total lipid | Dry film or in organic solvent, -20°C or lower | Indicate solvent composition upon submission |
| Feces (Optional) | ≥ 100 mg | -80°C | Use sterile containers; avoid preservatives |
Applications of Straight Chain Fatty Acids Assay Service
Microbiome Metabolite Profiling
Assess bacterial fermentation outputs in gut, oral, and skin microbiota studies.
Lipid Metabolism Research
Track fatty acid synthesis, elongation, and β-oxidation in metabolic pathway studies.
Nutritional Intervention Evaluation
Measure changes in fatty acid composition in response to dietary supplements or feeding regimes.
Cell Culture and In Vitro Models
Analyze extracellular and intracellular fatty acid levels in engineered or stimulated cell systems.
Environmental and Fermentation Monitoring
Quantify metabolic byproducts in bioprocess samples such as fermentation broth or bioreactor effluent.
Animal Model Metabolic Phenotyping
Profile fatty acid alterations in tissues and biofluids under genetic or environmental interventions.
Demo

PCA chart

PLS-DA point cloud diagram

Plot of multiplicative change volcanoes

Metabolite variation box plot

Pearson correlation heat map
FAQ of Straight Chain Fatty Acids Analysis Service
What are the detection limits for your analysis?
Detection limits typically range from 1 to 10 ng/mL depending on the specific fatty acid and instrument platform. Quantification is linear across at least 4–5 orders of magnitude.
Can you process complex biological samples such as feces or tissues?
Yes, we have optimized extraction protocols for challenging matrices including feces, liver, brain, and microbial fermentation media.
Do you provide absolute or relative quantification?
Both are available. Absolute quantification is achieved using isotopically labeled internal standards; relative quantification is also offered for untargeted or semi-targeted studies.
Is metabolite identification confirmed with standards?
Yes, all key straight chain fatty acids are confirmed using authentic chemical standards and validated retention times/mass spectra.
How should samples be prepared before shipment?
Samples should be aliquoted, labeled clearly, frozen at –80°C, and shipped on dry ice. Avoid repeated freeze-thaw cycles.
Can you help with method development or custom target panels?
Yes, we support custom assay design based on user-specified fatty acids, metabolic pathways, or experimental goals.
Do you offer isotope tracing studies for fatty acids?
Yes, we support ¹³C and ²H isotope labeling studies, including kinetic profiling and flux analysis using labeled substrates.
How do you ensure data quality and reproducibility?
Each batch includes QC samples, blanks, technical replicates, and spiked standards. We monitor instrument drift, matrix effects, and carryover.
What format is the final report delivered in?
Reports include raw and processed data in Excel or CSV, chromatograms, QC summaries, and optional visualizations (e.g., heatmaps, PCA, fold change plots).
Can I integrate the data into bioinformatics or statistical platforms?
Yes, all data is exportable in compatible formats for R, MetaboAnalyst, SIMCA, or other statistical software.
Do you support cross-species sample types (e.g., mouse, rat, human)?
Yes, our methods are validated across multiple species and biological matrices.
Are pooled or reference samples required for batch correction?
While not mandatory, pooled QCs or reference samples are highly recommended for studies involving multiple time points or treatment groups.
Learn about other Q&A.
Straight Chain Fatty Acids Analysis Service Case Study
Publications
Here are some publications in Lipidomics research from our clients:

- Loss of G0/G1 switch gene 2 (G0S2) promotes disease progression and drug resistance in chronic myeloid leukaemia (CML) by disrupting glycerophospholipid metabolism. 2022. https://doi.org/10.1002/ctm2.1146
- Glucosylceramide is essential for Heartland and Dabie bandavirus glycoprotein-induced membrane fusion. 2023. https://doi.org/10.1371/journal.ppat.1011232
- Evidence for phosphate-dependent control of symbiont cell division in the model anemone Exaiptasia diaphana. 2024. https://doi.org/10.1128/mbio.01059-24
- Prospective randomized, double-blind, placebo-controlled study of a standardized oral pomegranate extract on the gut microbiome and short-chain fatty acids. 2023. https://doi.org/10.3390/foods13010015
- White matter lipid alterations during aging in the rhesus monkey brain. 2024. https://doi.org/10.1007/s11357-024-01353-3
Reference
- Cheng, Hua, et al. "Selective desorption/ionization of sulfatides by MALDI-MS facilitated using 9-aminoacridine as matrix." Journal of lipid research 51.6 (2010): 1599-1609. https://doi.org/10.1194/jlr.D004077









