Pterins Analysis Service
Advanced pterin analysis service pinpoints trace-level cofactors and metabolites to de-risk R&D and manufacturing decisions.
Why Creative Proteomics?
- Trace-Level Detection: Sub-nanomolar LOD (0.1–0.5 nM) on the Agilent 6495C Triple Quad ensures you never miss low-abundance BH₄, BH₂, or neopterin.
- Wide Dynamic Range: Linear quantification across five orders of magnitude supports both baseline monitoring and high-concentration stress studies.
- Rock-Solid Reproducibility: < 5 % CV in 40-sample QC batches; matrix effect reduced by > 90 % with HILIC separation.
- Customizable Panels: Choose from 20 + pterins and related metabolites, or commission a strain- or matrix-specific assay.
Submit Your Request Now
×
Deliverables You Can Expect:
- PDF report with concentration data & BH₄/BH₂ ratios
- Annotated chromatograms & mass spectra (PNG/RAW)
- Excel workbook with calibrated peak areas and QC stats
- Method & instrument parameters sheet
- What We Provide
- Technology Platform
- Sample Requirement
- Demo
- Case
- FAQ
What Are Pterins?
Pterins are a group of heterocyclic compounds derived from pteridine, widely distributed in biological systems. These molecules function as critical cofactors and regulatory intermediates in diverse metabolic and biosynthetic pathways, including folate metabolism, nitric oxide synthesis, and the regulation of aromatic amino acid hydroxylases. Due to their biochemical significance and redox-sensitive nature, pterins are frequently studied in oxidative stress biology, fermentation quality control, and molecular pathway elucidation.
Structurally, pterins can exist in reduced, partially oxidized, or fully oxidized states, making their accurate detection and quantification technically demanding but highly informative.
Application-Oriented Analytical Support

Biochemical and Metabolic Research
Pterins serve as critical components in multiple enzymatic pathways. Accurate analysis supports studies in:
- Cofactor turnover and precursor quantification
- Oxidative stress-related compound mapping
- Systems biology and pathway integration research

Nutritional Product and Bioactive Ingredient Development
Pterins naturally occur in select botanicals and fermented products. Our platform is ideal for:
- Active compound profiling in nutritional supplements
- Quality assurance and lot-to-lot consistency studies
- Supporting internal R&D with verified composition data

Industrial Fermentation and Process Monitoring
In fermentation-based production systems, pterin levels may serve as biochemical markers for pathway control. Use cases include:
- Monitoring of engineered microbial pathways
- Metabolite tracking in large-scale bioreactors
- Precursor-product conversion efficiency evaluation
Pterins Analysis Service Offered by Creative Proteomics
- Total Pterin Quantification: Quantitative measurement of total pterin content, including both oxidized and reduced forms. Applicable to blood, plant extracts, fermentation samples, and more.
- Reduced/Oxidized Pterin Ratio (BH₄/BH₂): Accurate differentiation and quantification of BH₄ and BH₂ to assess redox balance. Suitable for redox biology and metabolic regulation studies.
- Neopterin & Biopterin Profiling: Targeted analysis of neopterin and biopterin levels to support botanical studies, ingredient quality control, and metabolic flow evaluation.
- Metabolic Pathway Coverage Panel: Simultaneous detection of multiple pterin-related metabolites (e.g., sepiapterin, monapterin) for comprehensive pathway analysis.
- Pterin Stability Analysis in Formulations: Evaluation of pterin compound stability under different storage, pH, or formulation conditions. Supports product development and shelf-life assessment.
- Customized Pterin Detection Panels: Tailored analyte panels designed for specific microbial strains, plant sources, or active compound screening based on client goals.
- Batch-to-Batch Quantitative Comparison: Comparative quantification of pterin compounds across production batches or treatment conditions to ensure quality consistency and process validation.
List of Detectable Pterins and Related Metabolites
| Compound | Category | Related Pathways / Significance |
|---|---|---|
| Tetrahydrobiopterin (BH₄) | Cofactor | Tyrosine/Tryptophan/Phenylalanine hydroxylation; NO synthesis |
| Dihydrobiopterin (BH₂) | Oxidized Form | BH₄ redox cycling; marker of oxidative stress |
| Neopterin | Immune Marker | Immune activation, inflammation, oxidative stress |
| Biopterin | Metabolite | Indicator of BH₄ metabolism status |
| Sepiapterin | Precursor | BH₄ biosynthesis intermediate |
| Xanthopterin | Catabolite | Pterin degradation product |
| 7,8-Dihydroneopterin | Intermediate | Early step in BH₄ biosynthesis |
| Pterin-6-carboxylic acid | Catabolite | Breakdown product of various pterins |
| Folates (THF, 5-MTHF, DHF, etc.) | Cofactors | One-carbon metabolism; nucleotide synthesis |
| 6-Hydroxymethylpterin | Intermediate | Folate and pterin metabolism |
| Formylpterin | Oxidized Product | Oxidative degradation product of folates and pterins |
| Lumazine derivatives | Precursor/Intermediates | Precursors in riboflavin and pterin pathways |
| Isoxanthopterin | Catabolite | Urinary excretion marker; pterin catabolism |
| 6-Carboxypterin | Catabolite | Oxidation product from pterin derivatives |
| 2-Amino-4-hydroxypteridine | Metabolite | Minor catabolite, oxidative stress marker |
| Monapterin | Cofactor | Insect and microbial metabolism; emerging interest in research |
| Dithiolene-bound molybdopterin | Cofactor | Molybdenum enzyme activity; relevant in microbial metabolism |
| Quinonoid dihydrobiopterin (qBH₂) | Reactive Intermediate | Short-lived intermediate during BH₄ redox cycling |
| Norbiopterin | Metabolite | BH₄ degradation product; potential oxidative stress indicator |
| 7,8-Dihydrobiopterin mono- and di-glucuronide | Conjugated Metabolites | Phase II metabolism of pterins in liver/kidney |
Methods and Instrumentation for Pterins Analysis
We integrate UHPLC systems—notably the Agilent 1260 Infinity II HPLC—coupled to high-performance Triple Quadrupole Mass Spectrometers such as the Agilent 6495C LC/MS. This configuration enables:
- Limits of Detection (LOD) as low as 0.1–0.5 nM for critical analytes like tetrahydrobiopterin (BH₄)
- Dynamic quantification across 4–5 orders of magnitude, suitable for diverse biological matrices
- Multiple Reaction Monitoring (MRM) acquisition, achieving high specificity for isomeric compounds
- Robust separation of highly polar pterins using HILIC chromatography columns, improving resolution and reducing matrix interference
For specific analytes exhibiting native fluorescence (e.g., neopterin, biopterin), we also deploy HPLC with Fluorescence Detection (FLD), achieving detection limits in the picomole range.

Agilent 6495C Triple Quadrupole (Figure from Agilent)

Agilent 7890B-5977A (Figure from Agilent)

Agilent 1260 Infinity II HPLC (Fig from Agilent)
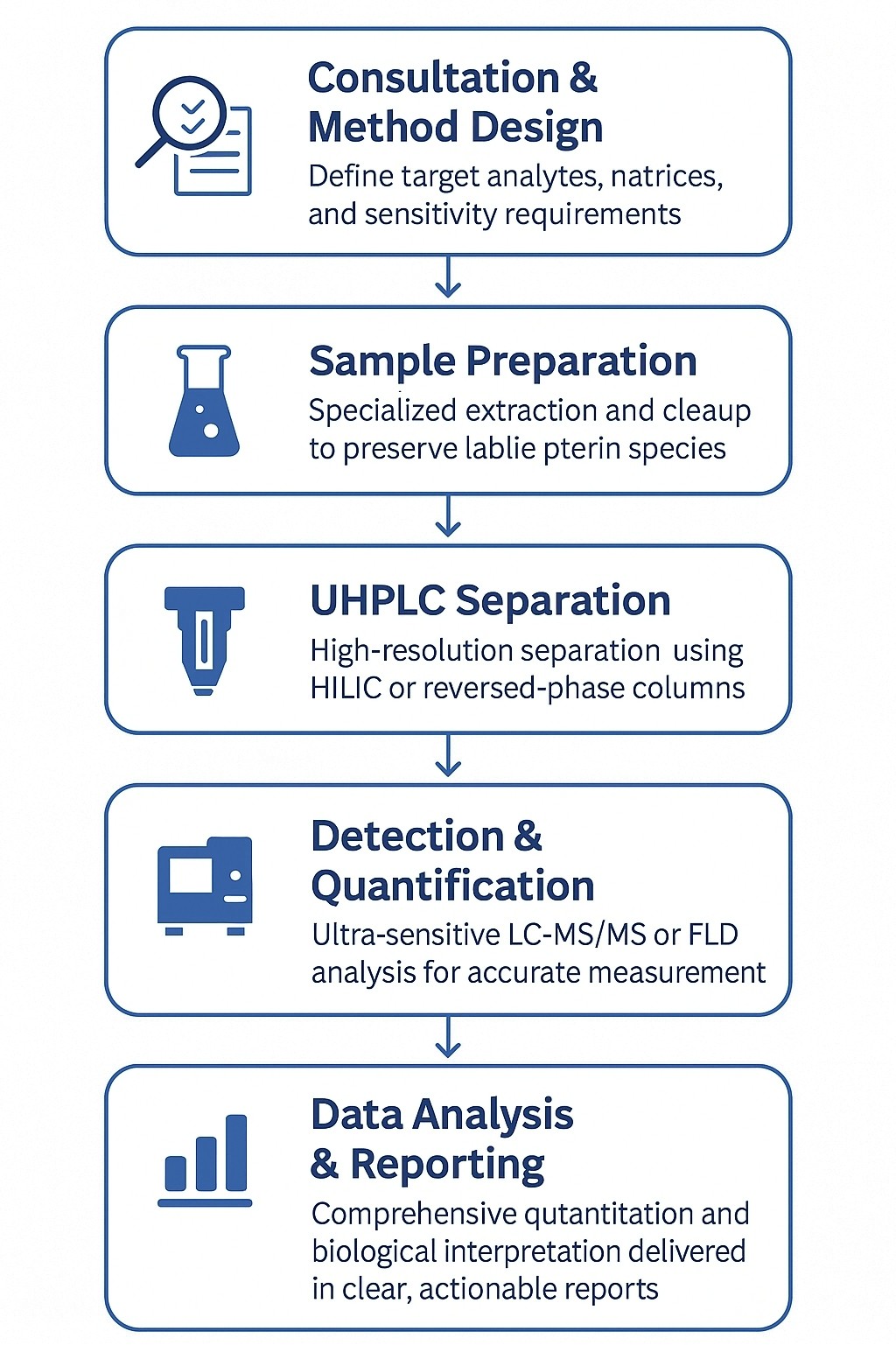
Our Pterins Assay Workflow
Sample Requirements for Pterins Analysis Service
| Sample Type | Minimum Volume / Amount | Container Type | Storage Conditions | Special Notes |
|---|---|---|---|---|
| Plasma / Serum | ≥ 100 µL | Polypropylene microtube | -80°C, protected from light | Use EDTA anticoagulant; avoid repeated freeze-thaw |
| Urine | ≥ 500 µL | Amber or foil-wrapped microtube | -80°C, protected from light | First morning sample preferred |
| Tissue (Animal / Plant) | ≥ 10 mg | Cryovial or sterile Eppendorf tube | Snap-frozen in liquid nitrogen, -80°C | Homogenize in cold buffer if extracting in-house |
| Cell Pellet | ≥ 1×10⁶ cells | Microtube with pellet | -80°C | Wash with cold PBS before freezing |
| Fermentation Broth | ≥ 1 mL | Sterile screw-cap tube | -20°C to -80°C | Filter to remove debris if possible |
| Food / Nutritional Matrix | ≥ 500 mg | Sealed, light-protected container | -20°C | Submit finely ground powder or homogenized slurry |
| Plant Extract | ≥ 200 µL | Amber glass or foil-wrapped tube | -80°C | Protect from oxidation during transport |
Demo Results

Representative HILIC-UHPLC-MS/MS workflow for pterin quantification. (A) Baseline-resolved chromatogram showing tetrahydrobiopterin (BH₄, tᴿ ≈ 1.8 min) and dihydrobiopterin (BH₂, tᴿ ≈ 2.3 min). (B) Product-ion spectrum of BH₄ (precursor m/z 240 → product ions m/z 166.1, 119.1) confirming compound identity and fragmentation pattern.
Pterins Analysis Service Case Study

Title: LC‑MS/MS Analysis of Cerebrospinal Fluid Metabolites in the Pterin Biosynthetic Pathway
Journal: JIMD Reports
Published: 2014
- Study Summary
- Background
- Methods
- Results
- Reference
This study developed and validated a rapid, sensitive LC‑MS/MS assay to quantify four pterin metabolites—BH₄, BH₂, neopterin, and sepiapterin—in cerebrospinal fluid (CSF). The method enables comprehensive screening and differential diagnosis of inherited BH₄ metabolism disorders.
Deficiencies in enzymes such as GTPCH, PTPS, DHPR, and SR cause inborn errors of tetrahydrobiopterin (BH₄) metabolism, leading to pediatric neurotransmitter disorders often accompanied by hyperphenylalaninemia. Traditional assays require separate tests for each metabolite, limiting efficiency.
- Standards/Internal Standards: BH₄, BH₂, neopterin, sepiapterin, and ^15N labeled analogs.
- Sample Prep: Mix 30 µL CSF with 210 µL internal standard solution, vortex, and inject.
- Chromatography: Reversed phase HPLC with phenyl-hexyl column, 10-min run, gradient from 95% aqueous to 100% methanol.
- Mass Spectrometry: AB Sciex 5500 QTRAP, +ESI mode, MRM transitions set for analytes and labeled counterparts.
- Performance: Linear range 3–200 nmol/L; intra-assay CV<6%, inter-assay CV <10%; recoveries 98–112% (except sepiapterin 75–85%).
- Validation: Compared to HPLC EC/fluorescence; strong correlation (BH₄ r=0.965; neopterin r=0.992).
![]() How Creative Proteomics Can Help?
How Creative Proteomics Can Help?
- Custom development of stable‑isotope dilution LC‑MS/MS assays for pterins or related metabolites.
- Sample preparation protocols compliant with clinical CSF handling.
- Full method validation (linearity, precision, accuracy, recovery).
- Comparative analysis against HPLC‑based reference methods.
- Ongoing assay performance monitoring and QC services.
The LC MS/MS method successfully distinguished between metabolic defects:
- SR deficiency: elevated BH₂ and sepiapterin, normal BH₄.
- DHPR deficiency: increased BH₂.
- PTPS deficiency: increased neopterin, low BH₄/BH₂.
- GTPCH deficiency (autosomal dominant): low BH₄/BH₂.
Sepiapterin was undetectable in controls and other disorders.
This assay enabled single-run differentiation of pterin pathway defects, facilitating targeted genetic testing and timely patient management.
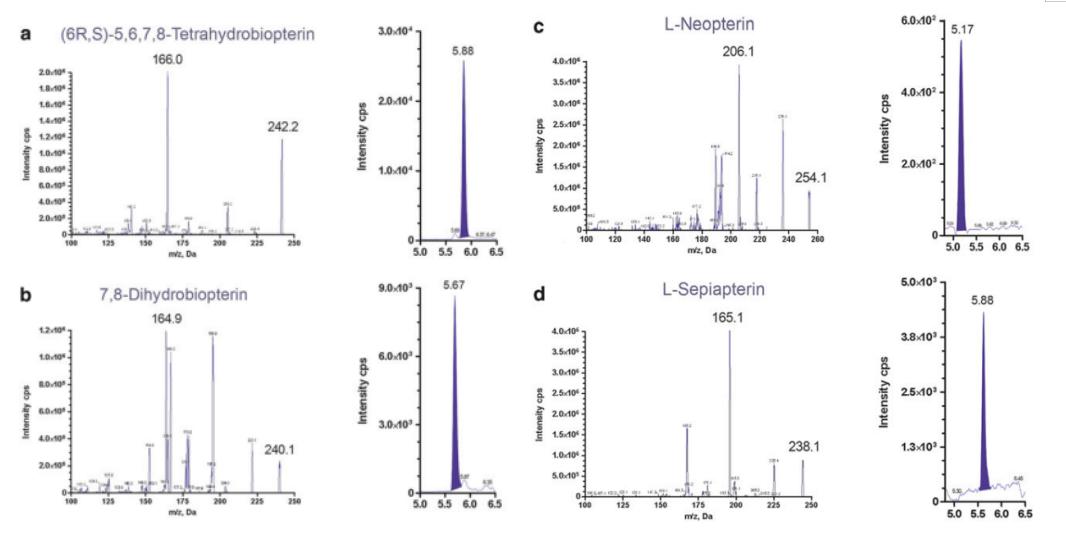 Product ion spectra of each pterin and a representative chromatograph of each metabolite in CSF. (a, BH4 ¼ 51 nM; b, BH2 ¼ 39 nM; c, neopterin ¼ 20 nM; and d, sepiapterin ¼ 23 nM). Spectra were generated with + ESI-MS/MS by infusion (10 mL/min) of pure standards (1 mM/L each)
Product ion spectra of each pterin and a representative chromatograph of each metabolite in CSF. (a, BH4 ¼ 51 nM; b, BH2 ¼ 39 nM; c, neopterin ¼ 20 nM; and d, sepiapterin ¼ 23 nM). Spectra were generated with + ESI-MS/MS by infusion (10 mL/min) of pure standards (1 mM/L each)
Reference
- Arning, Erland, and Teodoro Bottiglieri. "LC-MS/MS analysis of cerebrospinal fluid metabolites in the pterin biosynthetic pathway." JIMD Reports, Volume 29. Berlin, Heidelberg: Springer Berlin Heidelberg, 2014. 1-9. https://doi.org/10.1007/8904_2014_336
FAQ of Pterins Analysis Service
Can I send different biological matrices in the same project?
Yes. Our platform handles plasma, urine, tissues, cell pellets, fermentation broth, and botanical extracts within a single work order. We process each matrix under its own validated protocol to prevent cross-matrix interference and ensure comparable data.
How much sample do I need if I want the full 20 + metabolite panel?
For most fluid matrices, 150 µL is sufficient. Solid samples (tissue, food, plant material) require 15 mg. Providing a small surplus (≈20 %) allows us to repeat injections if any QC metric is out of tolerance.
Do you use isotope-labeled internal standards?
Absolutely. Each target pterin class is paired with a stable-isotope analogue, which corrects for extraction losses and matrix effects, giving you publication-grade quantitative accuracy.
What quality-control checkpoints are built into the run?
Every 10 injections we bracket a pooled QC sample; system suitability is verified at the beginning, midpoint, and end of the batch. Any drift beyond 15 % is automatically flagged for reinjection.
Can you integrate pterin data with a broader metabolomics study?
Yes. We supply raw and processed files in formats compatible with Compound Discoverer, Skyline, and MetaboAnalyst. Pathway annotations follow KEGG and MetaCyc identifiers, making downstream integrative analysis straightforward.
I work with genetically engineered microbes—can the panel be customized for novel pterin derivatives?
We routinely add new transitions within five working days once you provide the compound mass or a reference standard. Method extension is validated for linearity, precision, and carry-over before sample analysis resumes.
How do you protect sensitive project information?
All data are stored on encrypted servers with role-based access; raw files are retained for a minimum of two years and can be deleted sooner on written request. NDAs are supported but not required for strict confidentiality.
What happens if my sample concentration falls below the detection limit?
You'll receive a "below quantifiable limit" flag plus the estimated signal-to-noise ratio. At your option we can concentrate the extract (3–5 ×) and re-inject without additional service charges for the first repeat.
Can you scale up to hundreds of samples for a time-course fermentation study?
Our automated liquid-handling and dual-column UHPLC configuration supports 180+ injections per 24 hours, with real-time QC tracking to keep large-scale projects on schedule and within budget.
Learn about other Q&A about proteomics technology.
Publications
Here are some of the metabolomics-related papers published by our clients:

- Methyl donor supplementation reduces phospho‐Tau, Fyn and demethylated protein phosphatase 2A levels and mitigates learning and motor deficits in a mouse model of tauopathy. 2023. https://doi.org/10.1111/nan.12931
- A human iPSC-derived hepatocyte screen identifies compounds that inhibit production of Apolipoprotein B. 2023. https://doi.org/10.1038/s42003-023-04739-9
- The activity of the aryl hydrocarbon receptor in T cells tunes the gut microenvironment to sustain autoimmunity and neuroinflammation. 2023. https://doi.org/10.1371/journal.pbio.3002000
- Lipid droplet-associated lncRNA LIPTER preserves cardiac lipid metabolism. 2023. https://doi.org/10.1038/s41556-023-01162-4
- Inflammation primes the kidney for recovery by activating AZIN1 A-to-I editing. 2023. https://doi.org/10.1101/2023.11.09.566426
- Anti-inflammatory activity of black soldier fly oil associated with modulation of TLR signaling: A metabolomic approach. 2023. https://doi.org/10.3390/ijms241310634
- Plant Growth Promotion, Phytohormone Production and Genomics of the Rhizosphere-Associated Microalga, Micractinium rhizosphaerae sp. 2023. https://doi.org/10.3390/plants12030651






