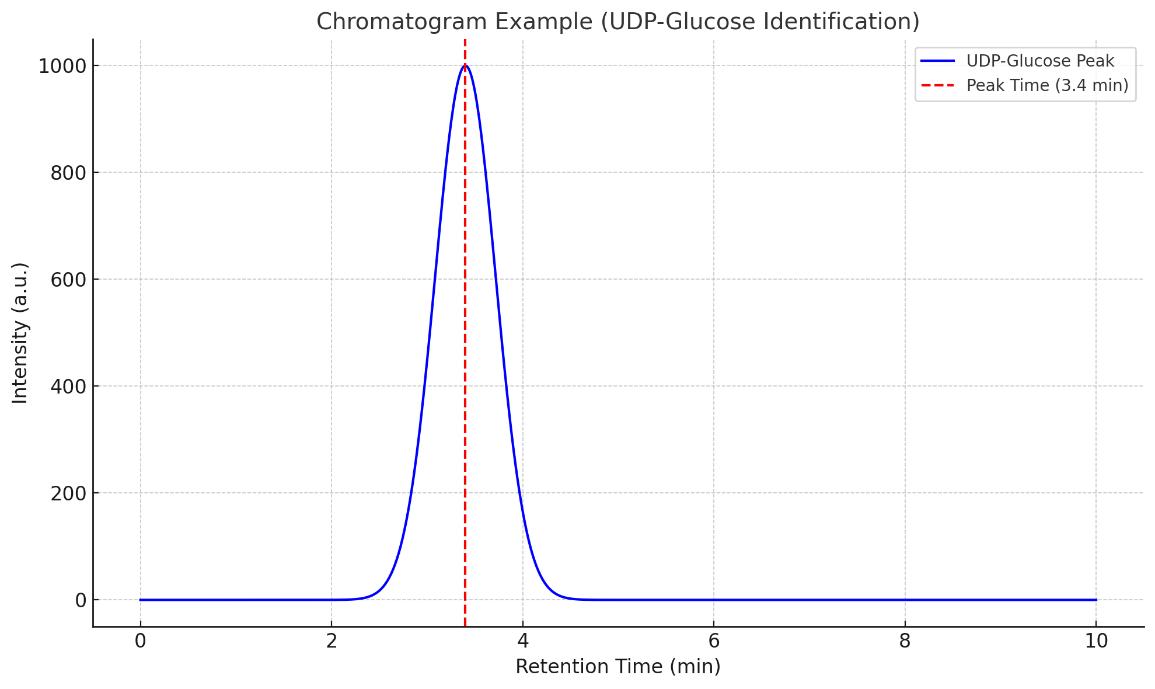
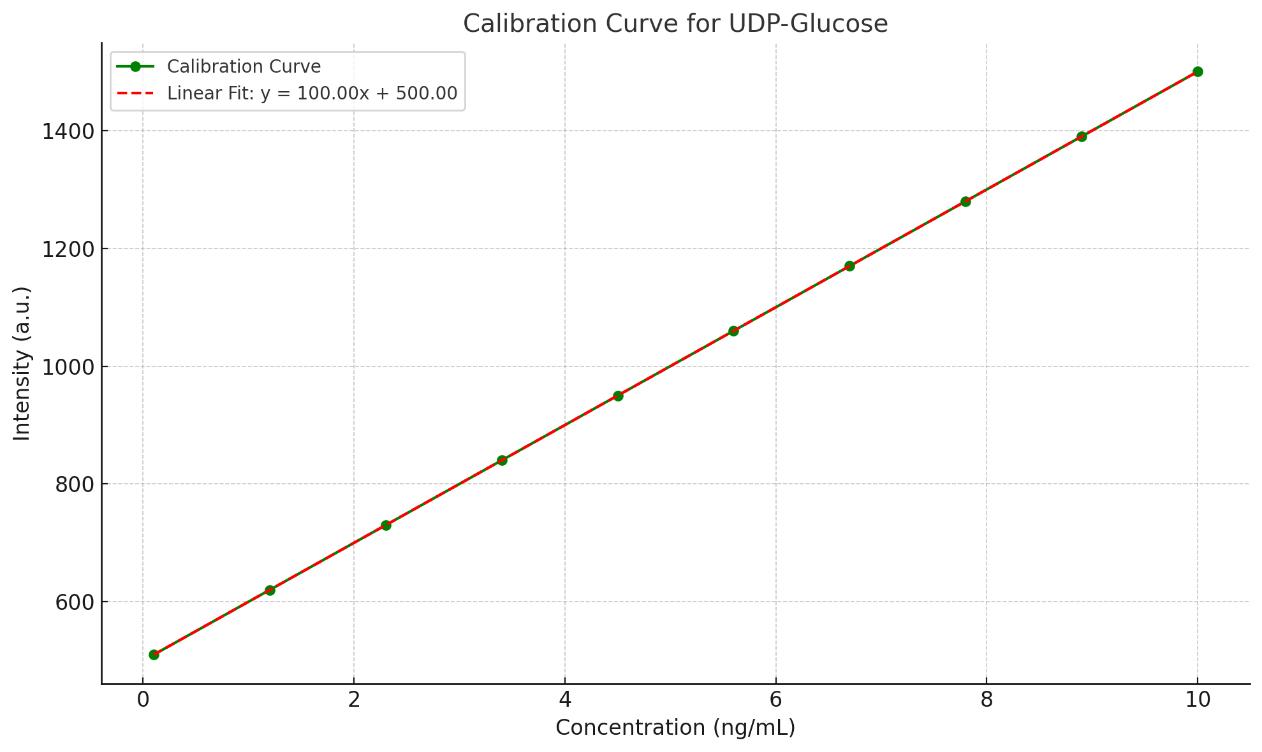
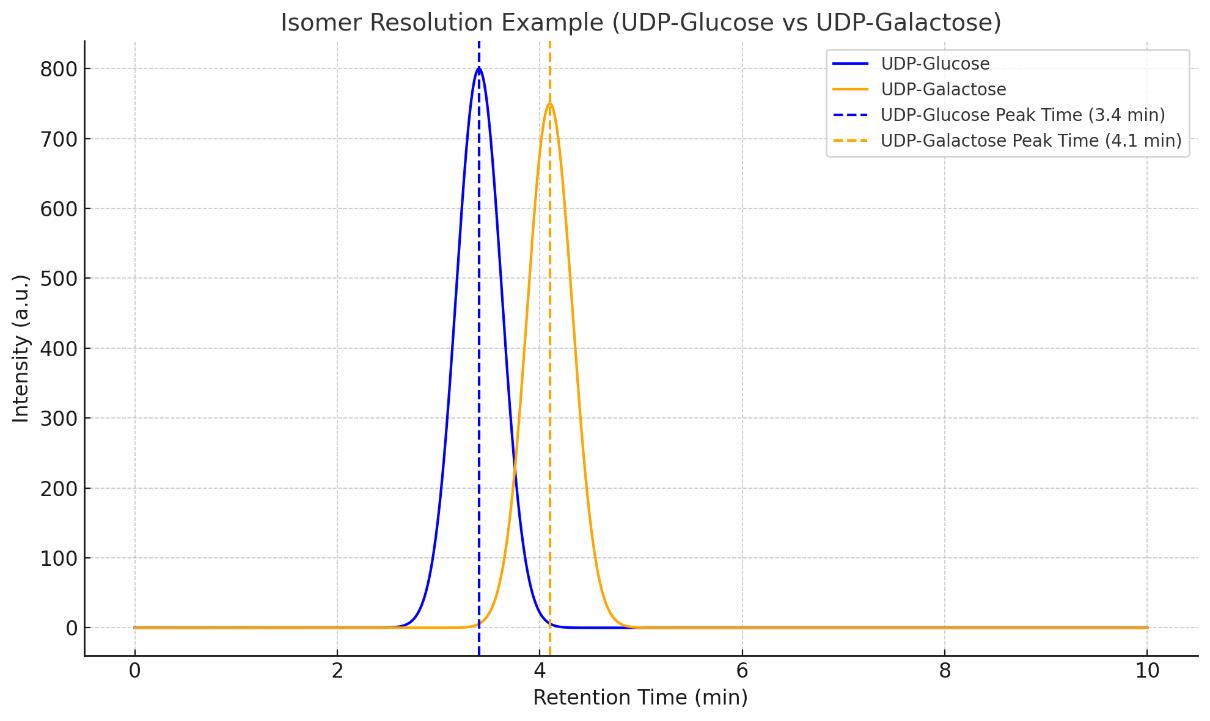
Nucleotide Sugars Analysis Service
Unlock critical insights into glycosylation pathways with Creative Proteomics' Nucleotide Sugars Analysis Service. Whether optimizing biopharma production, investigating disease biomarkers, or engineering metabolic pathways, precise profiling of nucleotide sugars empowers you to control glycan structures and ensure product consistency.
- Quantify 30+ nucleotide sugars with femtomole sensitivity
- Advanced LC-MS/MS and GC-MS platforms
- Absolute quantification with internal standards
- Supports diverse matrices: cells, tissues, biofluids, fermentation broths
- Data integrates seamlessly into glycomics and metabolomics workflows
Explore how our expertise can advance your research.
Submit Your Request Now
×
Deliverables You Can Expect:
- Detailed concentration reports (nmol/mg, or as requested)
- High-quality chromatograms and mass spectra
- Technical summary of methods and QC metrics
- Expert consultation on interpreting results
- What We Provide
- Technology Platform
- Sample Requirement
- Demo
- Case
- FAQ
What Are Nucleotide Sugars?
Nucleotide sugars are complex molecules consisting of a sugar moiety linked to a nucleotide. These molecules are crucial for glycosylation processes, where they serve as activated donors of sugar units in the formation of glycosidic bonds, which are essential for the synthesis of glycoproteins, glycolipids, and polysaccharides. Nucleotide sugars are involved in various biological processes such as protein folding, cell signaling, and the regulation of immune responses.
The most common nucleotide sugars include UDP-glucose, UDP-galactose, CMP-sialic acid, GDP-mannose, and others. These molecules are synthesized through complex enzymatic pathways and are vital for cellular homeostasis.
Why Analyze Nucleotide Sugars?
Enabling Control of Glycosylation Profiles
In biopharmaceutical manufacturing, glycan structures determine therapeutic protein efficacy, immunogenicity, and half-life. Measuring nucleotide sugar pools provides insight into intracellular availability of glycosylation precursors, critical for process optimization and quality control.
Driving Disease Research and Biomarker Discovery
Abnormal nucleotide sugar metabolism can signal disruptions in glycan biosynthesis linked to conditions such as cancer or metabolic disorders. Profiling these metabolites helps reveal molecular mechanisms underlying disease processes.
Supporting Metabolic Engineering and Synthetic Biology
Engineering of microbial or plant systems often targets nucleotide sugar pathways to enhance production of glycosylated biomolecules. Quantitative data guide strain design and process development.
Our Specialized Nucleotide Sugars Profiling Services
- Targeted Quantification: Accurate measurement of 30+ nucleotide sugars across diverse biological matrices.
- Rare Analyte Detection: Identification and confirmation of low-abundance or novel nucleotide sugars via high-resolution MS.
- Custom Method Development: Assays tailored to your unique glycosylation pathways or research goals.
- Data Integration: Results formatted for seamless inclusion in glycomics, metabolomics, and systems biology workflows.
List of Detected Nucleotide Sugars and Related Metabolites
| Nucleotide Sugar | Common Applications | Key Metabolic Pathways Involved | Detection Method |
|---|---|---|---|
| UDP-Glucose | Glycogen synthesis, glycosylation | Carbohydrate metabolism | HPLC, MS |
| UDP-Galactose | Glycosylation, lactose biosynthesis | Glycobiology, galactose metabolism | HPLC, MS |
| CMP-Sialic Acid | Sialylation of glycoconjugates | Cell signaling, immune response | HPLC, MS |
| GDP-Mannose | Glycosylation, fucose biosynthesis | Glycan biosynthesis | HPLC, MS |
| UDP-N-acetylglucosamine | Glycosylation, chondroitin synthesis | Glycobiology, extracellular matrix formation | HPLC, MS |
| UDP-N-acetylgalactosamine | Glycosylation, glycosaminoglycan biosynthesis | Extracellular matrix formation | HPLC, MS |
| GDP-Fucose | Glycosylation, fucosylation in glycoproteins | Immune response, cancer cell signaling | HPLC, MS |
| UDP-GlcNAc | Glycosylation, glycoprotein biosynthesis | Glycan synthesis, cellular signaling | HPLC, MS |
| CMP-Neu5Ac | Sialylation, glycolipid synthesis | Neuromodulation, immune system signaling | HPLC, MS |
| UDP-Xylose | Xylosylation in proteoglycan biosynthesis | Extracellular matrix biosynthesis | HPLC, MS |
Methods and Instrumentation for Nucleotide Sugars Analysis
LC-MS/MS Analysis
- System: Agilent 6495C Triple Quadrupole LC/MS
- Column: Waters XBridge BEH C18, 2.1 × 100 mm, 1.7 μm (or HILIC column for polar nucleotide sugars)
- Ionization: ESI (Electrospray Ionization), Positive Mode
- LOD: ~0.2 ng/mL
- Strengths: Exceptional sensitivity, isomer resolution, suited for complex matrices
 Agilent 6495C Triple quadrupole (Figure from Agilent)
Agilent 6495C Triple quadrupole (Figure from Agilent)
GC-MS Analysis
- System: Agilent 7890B GC coupled with 5977A MSD
- Derivatization: BSTFA (N,O-Bis(trimethylsilyl)trifluoroacetamide) is commonly used for converting nucleotide sugars to volatile derivatives.
- Column: DB-5MS UI, 30 m × 0.25 mm × 0.25 μm
- Strengths: Ideal for volatile derivatives and robust QC applications
 Agilent 7890B-5977A (Figure from Agilent)
Agilent 7890B-5977A (Figure from Agilent)
HPLC-FLD Analysis
- System: Agilent 1260 Infinity II HPLC with fluorescence detector
- Derivatization: o-Phthalaldehyde (OPA) or FMOC-Cl
- Excitation/Emission: 340/455 nm (for OPA derivatives)
- Strengths: Cost-effective, reliable for routine analysis
 Agilent 1260 Infinity II HPLC (Figure from Agilent)
Agilent 1260 Infinity II HPLC (Figure from Agilent)
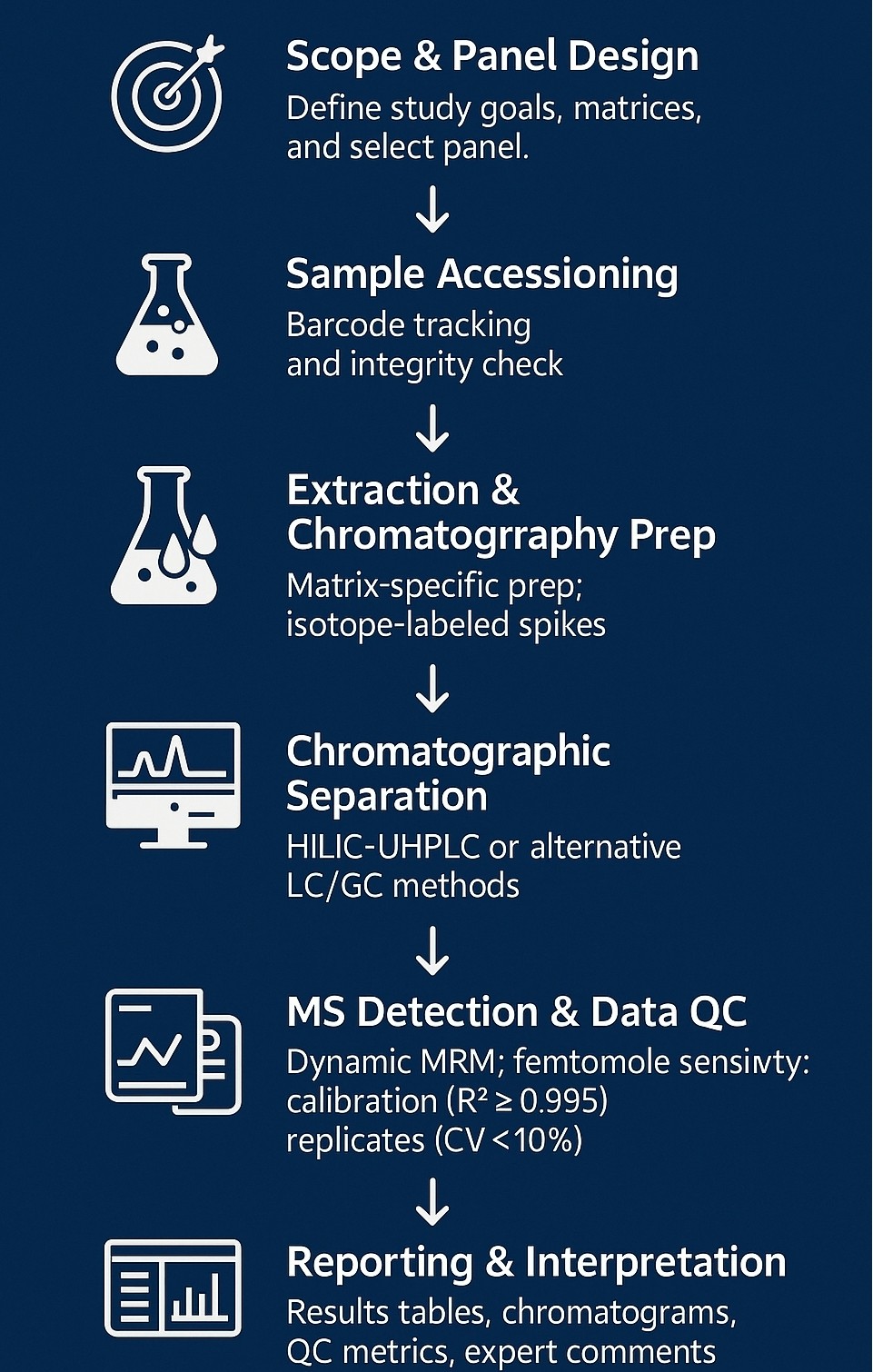
Analytical Workflow: Your Path to Reliable Nucleotide Sugars Data
1. Scope & Panel Design – Align study goals, matrices, and sensitivity needs; choose full or custom nucleotide-sugar panel.
2. Sample Accessioning – Barcode tracking and integrity checks secure chain-of-custody and quality.
3. Extraction & Internal Standards – Matrix-optimized prep with isotope-labeled spikes ensures accurate recovery.
4. Chromatographic Separation – HILIC-UHPLC as primary mode; complementary LC or GC for challenging analytes.
5. High-Sensitivity MS Detection – Dynamic MRM delivers femtomole-level detection and wide linear range.
6. Data Processing & QC – Matrix-matched calibration (R² ≥ 0.995), blanks, spikes, and replicates (CV < 10 %) safeguard data integrity.
7. Reporting & Interpretation – Concentration tables, annotated chromatograms, QC metrics, and expert insights packaged for easy review.
Sample Requirements for Nucleotide Sugar Analysis Service
| Sample Type | Volume Required | Matrix | Storage Conditions | Handling Instructions |
|---|---|---|---|---|
| Blood Plasma | 100 µL | Serum/Plasma | -80°C for long-term storage | Freeze immediately after collection |
| Cell Lysates | 200 µL | Mammalian/Plant Cells | -80°C or liquid nitrogen | Use protease inhibitors, avoid thawing/freeze |
| Tissue Homogenates | 200 mg | Animal Tissue | -80°C or liquid nitrogen | Homogenize in ice-cold buffers immediately |
| Urine | 500 µL | Urinary Tract | -80°C or liquid nitrogen | Collect in sterile containers, avoid contamination |
| Culture Media | 500 µL | Growth Media | 4°C for short-term, -80°C for long-term | Freeze if not analyzed immediately |
| Recombinant Proteins | 100 µg | Purified Proteins | -20°C or -80°C | Store in aliquots to avoid multiple freeze/thaw cycles |
Demo Results
Nucleotide Sugar Analysis Service Case Study

Title: The Arabidopsis Golgi-localized GDP-L-fucose transporter is required for plant development
Journal: Plant‐Environment Interactions
Published: 2024
- Study Summary
- Background
- Methods
- Results
- Reference
This study identifies GDP-fucose transporter 1 (GFT1) in Arabidopsis, which is critical for the transport of GDP-L-fucose into the Golgi lumen. The research reveals the essential role of GFT1 in plant growth and development, as GFT1-silenced plants exhibit severe growth defects due to reduced L-fucose in key cell wall polymers and N-glycans.
The Golgi apparatus plays a crucial role in the biosynthesis of cell wall polysaccharides and the glycosylation of proteins and lipids. GDP-sugars, including GDP-L-fucose, are vital for plant development, particularly in the fucosylation of xyloglucan and N-glycans. The transport of these nucleotide sugars into the Golgi is essential for proper glycosylation, yet little was known about the specific transporters responsible for this process in plants.
The study utilized proteo-liposome-based transport assays to examine the function of GFT1 in transporting GDP-L-fucose into the Golgi. Key steps include:
- Proteo-liposome Preparation: Microsomal proteins from Saccharomyces cerevisiae expressing GFT1 were reconstituted into liposomes to mimic biological membrane environments.
- Nucleotide Sugar Transport Assays: Proteo-liposomes were incubated with a mix of nucleotide sugar substrates (including GDP-L-fucose) and analyzed using LC-MS/MS. This allowed for precise measurement of transport activity.
Additionally, to explore the in vivo impact of GFT1 silencing, Arabidopsis plants were transformed with RNA interference constructs targeting GFT1. The expression levels of GFT1 and other GONST family members were quantified using qPCR, while the monosaccharide composition of cell wall extracts was determined through anion exchange chromatography.
![]() Creative Proteomics offers comprehensive Nucleotide Sugar analysis services to support research on glycosylation and plant development. Our capabilities include:
Creative Proteomics offers comprehensive Nucleotide Sugar analysis services to support research on glycosylation and plant development. Our capabilities include:
- Ultra-sensitive quantification of nucleotide sugars like GDP-Fuc, GDP-Man, and UDP-Glc
- High-resolution LC-MS/MS platforms, providing accurate measurements of nucleotide sugars in complex plant samples
- Comprehensive profiling of multiple nucleotide sugars to explore their roles in glycan biosynthesis and cell wall formation
- Customized analysis solutions tailored to meet the specific needs of plant glycosylation and development research
The study found that GFT1 preferentially transports GDP-L-fucose over other nucleotide sugars, with silenced GFT1 plants showing up to 80% reduced L-fucose content in their cell walls. This reduction significantly impacted cell wall structure, particularly in pectins and xyloglucans, and led to severe developmental defects. These findings confirm that GFT1 is the primary transporter for GDP-L-fucose in Arabidopsis, playing an indispensable role in plant growth and development.
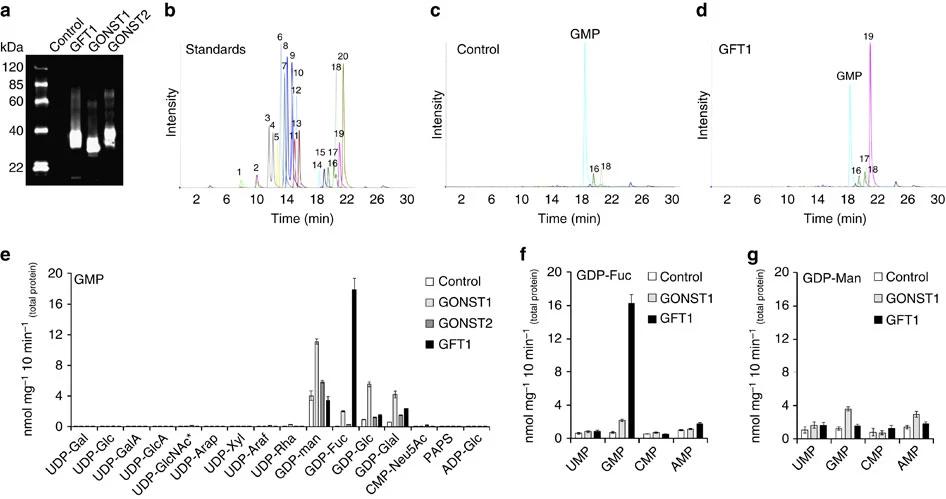 LC-MS/MS analysis of nucleotide sugar transport activities for GFT1 and other GONST members.
LC-MS/MS analysis of nucleotide sugar transport activities for GFT1 and other GONST members.
Reference
- Rautengarten, C., et al. "The Arabidopsis Golgi-localized GDP-L-fucose transporter is required for plant development. Nat. Commun. 7: 12119." 2016. https://doi.org/10.1038/ncomms12119
FAQ of Nucleotide Sugar Analysis Service
Can your assay differentiate between isomeric nucleotide sugars?
Yes. We use advanced chromatographic separation strategies, including HILIC-UHPLC and alternative orthogonal methods, to resolve isomeric nucleotide sugars with high confidence. Structural confirmation can also be supported by MS/MS fragmentation patterns.
What types of biological matrices can be analyzed?
Our service can handle diverse matrices such as cell lysates, tissue extracts, culture supernatants, serum, plasma, and other complex biological fluids. We provide guidance on appropriate sample volumes and preparation considerations for each matrix type.
How low can you detect nucleotide sugar concentrations?
Thanks to our dynamic MRM methods and optimized workflows, we routinely achieve femtomole-level sensitivity, allowing detection of low-abundance nucleotide sugars even in challenging matrices.
Do you provide assistance with study design for nucleotide sugar profiling?
Absolutely. We help clients define analytical goals, choose suitable panels (targeted or custom), and advise on experimental design to ensure data meets biological or regulatory research objectives.
Can you customize the nucleotide sugar panel to specific pathways or species?
Yes. We can develop targeted panels focusing on particular metabolic pathways or species-specific nucleotide sugars, supporting both standard research applications and specialized projects.
Is quantitation performed absolutely or relatively?
We offer absolute quantification using calibration curves prepared from authentic standards and stable isotope-labeled internal standards, ensuring high precision and accuracy in reported concentrations.
Do you support method validation for regulated studies?
Although our standard assays are designed for research use, we can provide additional validation parameters—such as accuracy, precision, linearity, matrix effects, and recovery studies—to support projects requiring higher regulatory standards (e.g., GLP-like studies).
Are nucleotide sugar reference standards required for my samples?
It's not mandatory for clients to supply standards; we maintain an extensive library of nucleotide sugar reference materials and internal standards. However, for rare or custom targets, collaboration on sourcing standards may be necessary.
Can the data support downstream biological pathway analysis?
Yes. Quantitative data from our assays can be integrated into metabolic pathway mapping tools, enabling clients to link nucleotide sugar profiles to broader biological or disease-related pathways.
Do you provide interpretation assistance beyond raw data reporting?
We deliver not only numerical results but also expert interpretation, helping clients understand biological implications, data trends, and potential next steps for research or product development.
Learn about other Q&A about proteomics technology.
Publications
Here are some of the metabolomics-related papers published by our clients:

- Methyl donor supplementation reduces phospho‐Tau, Fyn and demethylated protein phosphatase 2A levels and mitigates learning and motor deficits in a mouse model of tauopathy. 2023. https://doi.org/10.1111/nan.12931
- A human iPSC-derived hepatocyte screen identifies compounds that inhibit production of Apolipoprotein B. 2023. https://doi.org/10.1038/s42003-023-04739-9
- The activity of the aryl hydrocarbon receptor in T cells tunes the gut microenvironment to sustain autoimmunity and neuroinflammation. 2023. https://doi.org/10.1371/journal.pbio.3002000
- Lipid droplet-associated lncRNA LIPTER preserves cardiac lipid metabolism. 2023. https://doi.org/10.1038/s41556-023-01162-4
- Inflammation primes the kidney for recovery by activating AZIN1 A-to-I editing. 2023. https://doi.org/10.1101/2023.11.09.566426
- Anti-inflammatory activity of black soldier fly oil associated with modulation of TLR signaling: A metabolomic approach. 2023. https://doi.org/10.3390/ijms241310634
- Plant Growth Promotion, Phytohormone Production and Genomics of the Rhizosphere-Associated Microalga, Micractinium rhizosphaerae sp. 2023. https://doi.org/10.3390/plants12030651