Flavins Analysis Service
Creative Proteomics provides advanced flavin analysis services, including quantification of riboflavin, FMN, and FAD, profiling of related metabolites, and mapping of metabolic pathways. Our solutions help researchers investigate redox biology, optimize microbial fermentation, support synthetic biology design, and resolve cofactor imbalances affecting metabolic performance and enzyme activity.
Submit Your Request Now
×- What We Provide
- Advantage
- Workflow
- Technology Platforms
- Sample Requirements
- FAQ
- Publication
What are Flavins?
Flavins are a class of heterocyclic organic compounds based on pteridine, which include riboflavin (vitamin B₂) and its derivatives such as flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). These compounds play a crucial role in biological redox reactions, acting as essential cofactors for a wide range of flavoproteins. Flavins are involved in key metabolic processes including oxidative phosphorylation, amino acid catabolism, fatty acid oxidation, and the citric acid cycle.
Due to their central role in cellular metabolism and energy transfer, the precise quantification and profiling of flavins and their related metabolites is vital for biochemical research, industrial fermentation optimization, and metabolic engineering projects.
Flavins Analysis Service Offered by Creative Proteomics
- Flavin Quantification: Accurate measurement of riboflavin (Vitamin B₂), FMN, and FAD to assess cofactor levels and metabolic status.
- Flavin-Related Metabolite Profiling: Detection of degradation products like lumichrome and lumiflavin, as well as intermediates such as isoalloxazine derivatives, to study oxidative stress and metabolic pathways.
- Flavoprotein-Cofactor Interaction Studies: Analysis of flavin binding with flavoproteins and enzymes, and pathway mapping to explore enzyme function and regulation.
- Metabolic Flux Analysis: Use of stable isotope labeling (13C, 15N) to trace the flow of flavins and monitor time-course dynamics in metabolic networks.
- Comprehensive Pathway Metabolomics: Full profiling of flavin metabolism, identifying key intermediates and associated metabolites to support pathway and systems biology studies.
- Microbial and Plant Flavins Analysis: Flavin analysis in microbial fermentation and plant extracts to study their role in stress response, metabolic optimization, and productivity.
- Customized Services: Tailored panels and consultative research services, including specialized flavin metabolites and custom experimental design support.
- Industrial and Biotech Applications: Optimizing fermentation processes and examining flavin effects on biocatalysis and enzyme activity for improved production yields.
List of Detected Flavins and Related Metabolites
| Detected Compound | Metabolic Pathway | Related Metabolites |
|---|---|---|
| Riboflavin (Vitamin B₂) | Riboflavin metabolism | Lumichrome, Lumiflavin, Riboflavin 5'-phosphate |
| Flavin Mononucleotide (FMN) | Electron transport, Redox biology | FMN degradation products (e.g., 8-hydroxy-FMN) |
| Flavin Adenine Dinucleotide (FAD) | Oxidative phosphorylation, TCA cycle | FADH₂ (reduced form), FAD degradation products |
| Lumichrome | Riboflavin degradation, oxidative stress | - |
| Lumiflavin | Photodegradation, oxidative stress | - |
| Isoalloxazine Ring Compounds | Flavoprotein interactions | Flavodoxin, reduced flavodoxin |
| 8-Hydroxyflavins | Oxidative stress marker, degradation | 8-Hydroxyflavin derivatives |
| Flavodoxin | Anaerobic electron transfer, bacterial systems | Reduced flavodoxin, flavodoxin precursors |
| Isoalloxazine Derivatives | Flavoprotein-related pathways | Isoalloxazine-derivative cofactors (e.g., FMN, FAD) |
| 8-Hydroxy-FAD | FAD degradation, oxidative stress | 8-Hydroxyflavin, other oxidized flavins |
| Riboflavin 5'-Phosphate | Riboflavin metabolism, phosphorylation | FMN, FAD |
| Flavin-Related Intermediates | TCA cycle, electron transport | 5'-Phospho-FMN, 5'-Phospho-FAD |
Advantages of Flavins Assay
- Ultra-Sensitive Quantification: Detection limits reach as low as 0.1 ng/mL for riboflavin and its derivatives, enabling accurate analysis even in trace samples.
- High Reproducibility: Our validated protocols ensure <5% CV (coefficient of variation) across replicate samples.
- Broad Dynamic Range: Linear quantification is maintained across a concentration range of 0.1 ng/mL to 10 µg/mL.
- Multi-Matrix Compatibility: Compatible with complex matrices such as serum, plasma, cell lysates, microbial fermentation broth, plant extracts, and more.
- Comprehensive Coverage: Detection and quantification of over 25 flavin compounds and related metabolites, enabling full pathway analysis.
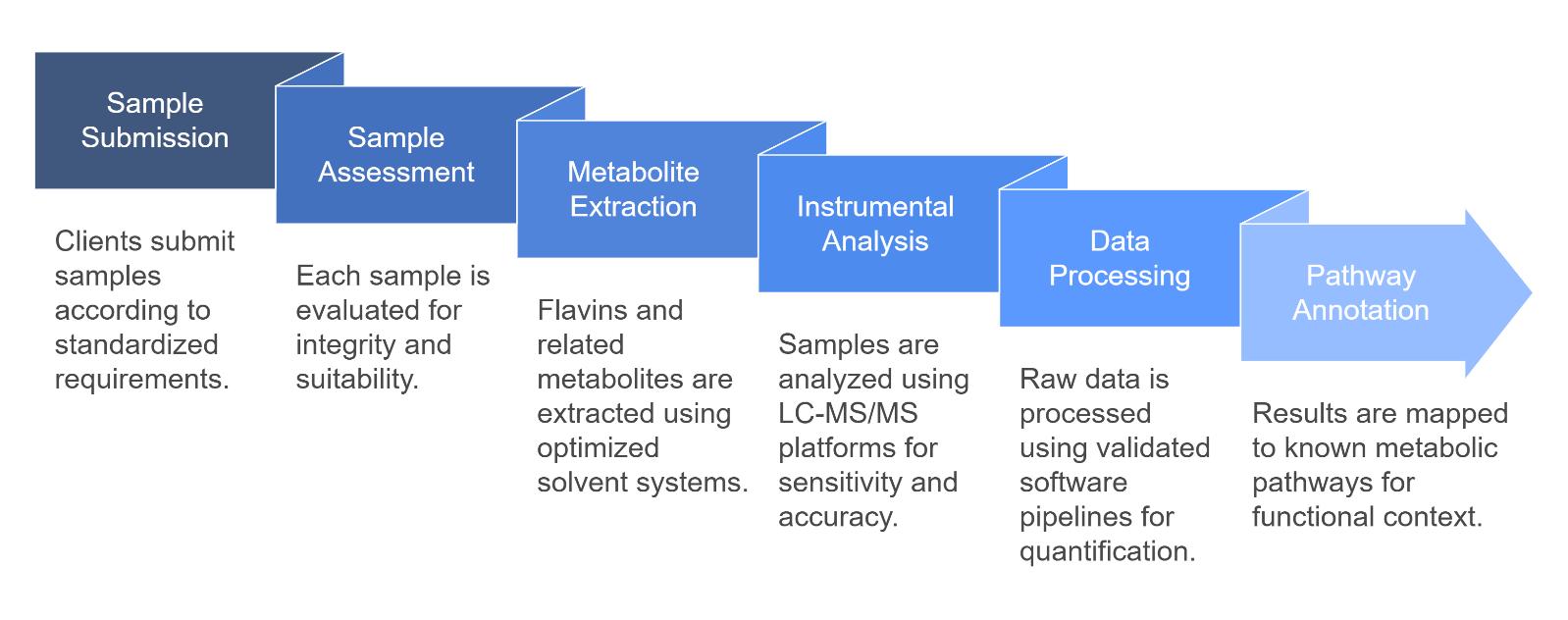
Workflow for Flavins Analysis Service
Technology Platform for Flavins Analysis Service
- Agilent 6495C Triple Quadrupole LC-MS/MS: For high-sensitivity quantification of flavins and metabolites, offering detection limits down to 0.1 ng/mL.
- AB Sciex Triple Quad™ 6500+: Used for targeted quantitation of flavins and their derivatives with high throughput and multiplexing ability.
- Agilent 1290 Infinity II UPLC System: Provides optimal separation and resolution of flavins for precise identification and quantification.

Agilent 6495C Triple quadrupole (Figure from Agilent)

SCIEX Triple Quad™ 6500+ (Figure from Sciex)

Agilent 1260 Infinity II HPLC (Fig from Agilent)
Sample Requirements for Flavins Analysis Service
| Sample Type | Minimum Volume/Mass Required |
|---|---|
| Serum / Plasma | ≥ 100 µL |
| Whole Blood | ≥ 200 µL |
| Cell Culture Supernatant | ≥ 200 µL |
| Cell Pellet | ≥ 1 × 10⁶ cells |
| Microbial Culture | ≥ 1 mL |
| Plant Tissue | ≥ 50 mg |
| Animal Tissue (e.g., liver) | ≥ 100 mg |
Applications of Flavins Assay Service
Microbial Metabolism Studies
Analyze flavin dynamics in bacteria and fungi to understand energy metabolism and redox balance.
Strain Engineering & Fermentation Optimization
Monitor flavin levels in engineered strains to enhance productivity and fermentation efficiency.
Plant Physiology & Stress Response
Profile flavins in plant tissues to study their roles in oxidative stress, growth, and light response.
Food and Nutritional Research
Quantify riboflavin and derivatives in food products and biological samples for nutritional evaluation and quality control.
Synthetic Biology Pathway Design
Evaluate flavin cofactor requirements and flux in synthetic pathways for metabolic engineering.
Redox Biology & Cofactor Analysis
Investigate flavin-dependent redox processes in cellular systems and enzyme catalysis.
Demo

HPLC-UV/Vis and HPLC-MS analysis of isolated flavin derivatives as side products of the bioluminescent reaction (Brodl, Eveline, et al. 2020).
FAQ of Flavins Analysis Service
Do flavins require light-protected handling during sample prep?
Yes. Flavins are photosensitive compounds. All extraction and processing steps are performed under low-light or amber conditions to preserve sample integrity.
Can I submit samples in organic solvents (e.g., methanol or acetonitrile)?
Yes, we accept samples preserved in solvents—simply note the solvent type during submission. Avoid solvents that interfere with flavin stability (e.g., strong acids).
Is technical replication included in the service?
Yes, each sample is analyzed in triplicate to ensure reproducibility. Custom replication requests (e.g., n=5) are available.
What is the difference between absolute and relative quantification?
Absolute quantification reports exact concentrations (e.g., ng/mL) using calibration curves, while relative quantification compares fold changes between groups.
Can you integrate flavin data with other metabolomics datasets?
Yes, we provide cross-platform data alignment (e.g., linking flavin levels to amino acid or lipid profiles) upon request.
Can you analyze flavins in extracellular media (e.g., cell culture supernatant)?
Yes, we validate methods for extracellular fluids, including media, urine, and fermentation broths.
How do you address matrix interference in complex samples (e.g., blood)?
We employ matrix-matched calibration curves and isotope dilution to correct for ion suppression/enhancement effects.
Can I request raw mass spectrometry files for independent analysis?
Raw data files (.raw, .wiff) are provided at no extra cost, compatible with software like Skyline or MaxQuant.
Learn about other Q&A.
Flavins Analysis Service Case Study
Publications
Here are some publications in Metabolomics research from our clients:

- The Suppression of the KRAS G12D-Nrf2 Axis Shifts Arginine into the Phosphocreatine Energy System in Pancreatic Cancer Cells. 2023. https://doi.org/10.1016/j.isci.2023.108566
- Insulin resistance does not impair mechanical overload-stimulated glucose uptake, but does alter the metabolic fate of glucose in mouse muscle. 2020. https://doi.org/10.3390/ijms21134715
- Overexpression of maize ZmLOX6 in Arabidopsis thaliana enhances damage-induced pentyl leaf volatile emissions that affect plant growth and interaction with aphids. 2023. https://doi.org/10.1093/jxb/erac522
- Effect of arabinogalactan on the gut microbiome: A randomized, double-blind, placebo-controlled, crossover trial in healthy adults. 2021. https://doi.org/10.1016/j.nut.2021.111273
- Function and regulation of a steroidogenic CYP450 enzyme in the mitochondrion of Toxoplasma gondii. 2023. https://doi.org/10.1371/journal.ppat.1011566
Reference
- Brodl, Eveline, et al. "The impact of LuxF on light intensity in bacterial bioluminescence." Journal of Photochemistry and Photobiology B: Biology 207 (2020): 111881. https://doi.org/10.1016/j.jphotobiol.2020.111881








