Cholestanol Analysis Service
Creative Proteomics offers high-precision cholestanol analysis using advanced LC-MS/MS and GC-MS technologies. We provide comprehensive sterol profiling, metabolic pathway mapping, and lipid research services. Our solutions help researchers investigate sterol dysregulation, monitor metabolic disorders, and optimize lipidomic studies. With unmatched sensitivity and reproducibility, we ensure accurate results for both routine analysis and complex research needs.
Submit Your Request Now
×- What We Provide
- Advantage
- Workflow
- Technology Platform
- Sample Requirements
- FAQ
- Publication
What is Cholestanol?
Cholestanol is a 5α-saturated derivative of cholesterol, structurally similar yet biologically distinct. As a secondary bile alcohol formed via microbial or enzymatic hydrogenation of cholesterol, cholestanol is minimally present in healthy individuals but is markedly elevated in certain metabolic disorders—most notably in cerebrotendinous xanthomatosis (CTX), a rare autosomal recessive lipid storage disease. Unlike cholesterol, which is integral to membrane structure and precursor for steroid hormones, cholestanol has limited physiological roles and is considered a metabolic biomarker for sterol dysregulation.
Cholestanol vs. Cholesterol
Cholestanol and cholesterol are structurally related sterols, but they differ significantly in their chemical properties and biological behavior. Cholestanol is a saturated sterol lacking the 5,6-double bond present in cholesterol, which alters its physical characteristics, including solubility and membrane fluidity modulation.
| Parameter | Cholesterol | Cholestanol |
|---|---|---|
| Molecular Formula | C₂₇H₄₆O | C₂₇H₄₈O |
| Core Structure | Δ5-unsaturated steroid nucleus | Saturated steroid nucleus |
| Functional Group | 3β-hydroxyl group | 3β-hydroxyl group |
| Membrane Interaction | Maintains fluidity and integrity | Lower integration into lipid bilayers |
| Biosynthetic Origin | Synthesized from acetyl-CoA | Derived from cholesterol hydrogenation |
| Physical Properties | Amphipathic; more rigid | More hydrophobic; less rigid |
Cholestanol Analysis Service Offered by Creative Proteomics
- Quantitative Measurement of Cholestanol Levels: High-throughput and precise quantification of cholestanol in biological samples (plasma, serum, cerebrospinal fluid (CSF), liver tissue, etc.) using advanced LC-MS/MS.
- Cholestanol to Cholesterol Ratio Analysis: Evaluation of the cholestanol/cholesterol ratio, a valuable metric for understanding sterol imbalances in metabolic conditions and dietary assessments.
- Comprehensive Lipid Profiling: Simultaneous quantification of cholestanol and related sterols (such as cholesterol, campesterol, sitosterol) to provide a holistic view of lipid metabolism and sterol distribution.
- Sterol Metabolite Profiling: Profiling of additional sterol metabolites, including 7α-hydroxycholestanol, desmosterol, lathosterol and other oxysterols and bile acid intermediates.
- Custom Metabolic Pathway Analysis: Identification and quantification of cholesterol-derived metabolites and sterols in specific metabolic pathways (e.g., bile acid synthesis, cholesterol biosynthesis).
- Sterol Analysis for Rare Diseases: Tailored services for detecting abnormal cholestanol levels linked to specific metabolic disorders, such as cerebrotendinous xanthomatosis (CTX), among other rare autosomal recessive conditions.
- Pathway Mapping and Interpretation: In-depth biochemical pathway analysis and interpretation of cholestanol metabolism, using our proprietary databases to link sterol concentrations to metabolic pathways, providing insights into physiological conditions and disease mechanisms.
List of Detected Cholestanol and Related Metabolites
| Analyte | Metabolic Pathway | Significance | Detection Limit |
|---|---|---|---|
| Cholestanol | Cholesterol Hydrogenation | Marker of sterol dysregulation, key in CTX and other sterolopathies | <0.5 ng/mL |
| Cholesterol | Cholesterol Biosynthesis | Essential membrane component, precursor for steroid hormones | <1.0 ng/mL |
| 7α-Hydroxycholestanol | Bile Acid Synthesis (CYP7A1 Pathway) | Bile acid precursor, involved in cholesterol homeostasis | <0.8 ng/mL |
| 27-Hydroxycholesterol | Oxysterol Synthesis (CYP27A1 Pathway) | Important signaling molecule in cholesterol metabolism and atherosclerosis | <0.7 ng/mL |
| Lathosterol | Cholesterol Biosynthesis | Intermediate in the Bloch pathway of cholesterol biosynthesis | <1.2 ng/mL |
| Desmosterol | Cholesterol Biosynthesis (Bloch Pathway) | Precursor to cholesterol, often elevated in metabolic conditions | <1.5 ng/mL |
| Campesterol | Phytosterol Absorption Pathway | Phytosterol; used as a biomarker for sterol intake from diet | <1.0 ng/mL |
| Sitosterol | Phytosterol Metabolism | Phytosterol; linked to sitosterolemia, a rare metabolic disorder | <1.0 ng/mL |
| Stigmasterol | Phytosterol Metabolism | Plant sterol; plays role in modulating lipid metabolism | <1.5 ng/mL |
| Cholest-5-en-3β-ol | Cholesterol Biosynthesis (Desmosterol Pathway) | Intermediate sterol; implicated in cholesterol and sterol metabolism | <1.2 ng/mL |
| 4β-Hydroxycholesterol | Cytochrome P450 (CYP3A4) Metabolism | Marker for CYP3A4 enzyme activity, linked to steroid metabolism | <0.9 ng/mL |
| Oxysterols (e.g., 24S-OH-cholesterol) | Cholesterol Oxidation Pathway (CYP46A1) | Oxidized cholesterol derivatives, involved in neurodegenerative diseases | <1.0 ng/mL |
| Cholest-7-en-3β-ol | Cholesterol Hydrogenation | Metabolite of cholesterol, used for sterol profiling in lipid metabolism studies | <1.0 ng/mL |
Advantages of Cholestanol Assay
- High Sensitivity: Detection limit as low as 0.5 ng/mL for cholestanol and<0.8 ng/mL for related metabolites.
- Excellent Reproducibility:<5% intra-assay CV and <8% inter-assay CV for reliable results.
- Comprehensive Profiling: Simultaneous analysis of up to 30 sterols and metabolites in a single assay.
- Scalable Capacity: Process up to 500 samples per batch for high-throughput studies.
- Accurate Metabolite Quantification: High precision with stable isotope-labeled internal standards.
- Advanced Data Reporting: Detailed metabolic pathway analysis and results in multiple formats.
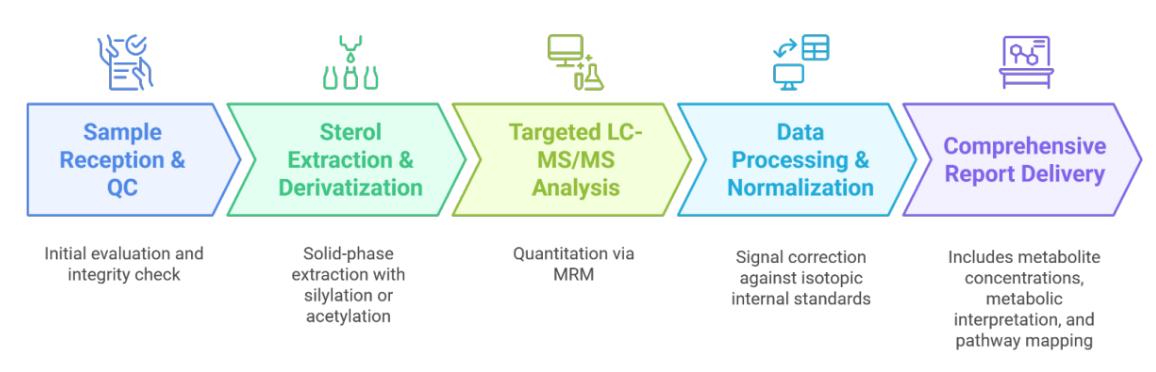
Workflow for Cholestanol Analysis Service
Technology Platform for Cholestanol Analysis Service
Sciex QTRAP® 6500+ (LC-MS/MS): Provides ultra-sensitive quantification with multiple reaction monitoring (MRM) for precise cholestanol analysis in complex biological samples.
Agilent 7890B-5977A (GC-MS): Ideal for analyzing derivatized cholestanol and related metabolites, offering high resolution for volatile and semi-volatile sterols.
Agilent 1260 Infinity II HPLC: Used for separation and quantification of non-volatile sterols, paired with MS for enhanced accuracy and resolution.

SCIEX Triple Quad™ 6500+ (Figure from Sciex)

Agilent 7890B-5977A (Figure from Agilent)

Agilent 1260 Infinity II HPLC (Fig from Agilent)
Sample Requirements for Cholestanol Analysis Service
| Sample Type | Minimum Volume Required | Notes |
|---|---|---|
| Plasma/Serum | ≥200 µL | Use EDTA or heparin; avoid hemolysis |
| CSF | ≥100 µL | Preferably frozen immediately post-collection |
| Liver Tissue | ≥30 mg | Snap-frozen; shipped on dry ice |
| Cultured Cells | ≥1×10⁶ cells | Washed, frozen cell pellet preferred |
| Fecal Samples | ≥100 mg | Sterile container; dry ice shipping |
Applications of Cholestanol Assay Service
Lipidomics Research
Provides insights into sterol metabolism, helping understand lipid-related biological processes.
Metabolic Disorder Studies
Essential for studying diseases like cerebrotendinous xanthomatosis (CTX) and other sterol metabolic disorders.
Nutritional Lipid Analysis
Measures sterol intake and metabolism, aiding in dietary studies and nutrition science.
Environmental Research
Used in monitoring plant sterol levels in ecosystems for ecological and environmental health studies.
Pharmacokinetics
Supports the analysis of sterol absorption and metabolism in drug development studies.
Neurobiology
Investigates the role of sterols like cholestanol in neurodegenerative diseases and brain lipid homeostasis.
FAQ of Cholestanol Analysis Service
What sample types are required for cholestanol analysis?
We accept plasma, serum, cerebrospinal fluid (CSF), liver tissue, and cultured cells.
How much sample is needed for analysis?
Typically, 100 µL of plasma or serum is sufficient for most analyses, though larger samples may be required for tissue analysis.
What is the turnaround time for cholestanol analysis?
Standard processing time is 7-10 business days, with expedited services available for urgent projects.
Can you analyze cholestanol levels in multiple samples at once?
Yes, we can process up to 500 samples per batch, making it ideal for high-throughput studies.
What is the detection limit for cholestanol?
We can detect cholestanol at levels as low as 0.5 ng/mL, ensuring accurate measurement even in trace amounts.
How accurate are the results for cholestanol analysis?
Our results are highly reproducible, with<5% intra-assay CV and <8% inter-assay CV, ensuring precision and reliability.
Can I receive the data in different formats?
Yes, results can be delivered in CSV, Excel, or PDF formats to suit your research needs.
Do I need to prepare or process the samples before sending them?
No pre-treatment is necessary; we recommend storing biological samples at –80°C and shipping them on dry ice to preserve integrity.
Can I combine cholestanol analysis with other sterol or lipid analyses?
Yes, we offer customizable panels that include cholesterol, phytosterols, oxysterols, and other lipids in a single run.
How are cholestanol and related metabolites extracted?
We use solid-phase extraction (SPE) and/or liquid-liquid extraction (LLE) depending on sample type and matrix complexity.
Is method validation data available for publication use?
Yes, we can provide method validation parameters (LOD, LOQ, CVs, recovery rate) upon request for research publications.
What quality controls are included in the analysis?
Each batch includes internal standards, blank samples, and QC replicates to ensure data accuracy and consistency.
Do you provide interpretation or pathway mapping of the results?
Yes, we offer basic metabolic pathway annotation and optional bioinformatics support upon request.
Can I ship international samples for analysis?
Yes, we accept global shipments, but please contact us for customs documentation and shipping conditions.
Learn about other Q&A.
Cholestanol Analysis Service Case Study

Title: Assays of plasma dehydrocholesteryl esters and oxysterols from Smith-Lemli-Opitz syndrome patients
Journal: Journal of Lipid Research
Published: 2013
- Background
- Materials & Methods
- Results
Smith-Lemli-Opitz Syndrome (SLOS) is a neurodevelopmental disorder caused by mutations in the DHCR7 gene, which encodes 3β-hydroxysterol-Δ7-reductase—an enzyme responsible for converting 7-dehydrocholesterol (7-DHC) to cholesterol. Due to this enzymatic defect, patients with SLOS show elevated levels of 7-DHC and 8-dehydrocholesterol (8-DHC), both of which are highly prone to lipid peroxidation. This oxidative instability can lead to the formation of cytotoxic oxysterols, contributing to disease pathology. The study addresses the need for accurate, oxidation-minimizing assays to analyze sterols and their esters in plasma, presenting HPLC/MS techniques to quantify these metabolites and identify oxysterols as potential biomarkers for disease severity and therapeutic response.
Materials:
Chemicals were sourced primarily from Sigma-Aldrich and Thermo Fisher. Synthesized deuterated sterols and oxysterols were used as internal standards. NH₂-SPE cartridges and HPLC-grade solvents were utilized.
Plasma Collection:
Blood was collected in EDTA tubes, protected from light, and treated with BHT and TPP to prevent oxidation. Plasma was stored at −80°C.
Total Sterol Analysis (GC):
Plasma lipids were extracted using chloroform-methanol with antioxidants, hydrolyzed with KOH, derivatized to trimethylsilyl ethers, and analyzed via GC-FID using a specified temperature gradient.
Free Sterols & Ester Analysis (HPLC-MS/MS):
Samples were extracted under red light, spiked with deuterated standards, and analyzed by reversed-phase HPLC-MS/MS with APCI-SRM detection.
Oxysterol Analysis:
Samples were spiked with d7-7-ketocholesterol, extracted, hydrolyzed, and analyzed using normal-phase HPLC-MS/MS.
Plasma sterols and their esters in SLOS patients were analyzed using LC-MS/MS with deuterated internal standards. Cholesterol and its esters were identified by fragment ion m/z = 369; 7-DHC and 8-DHC esters by m/z = 367. Chromatographic separation allowed distinction between 7-DHC and 8-DHC esters, with 7-DHC esters eluting earlier. The most abundant cholesteryl esters were linoleate, oleate, palmitate, and arachidonate. Quantitative analysis revealed variable esterification ratios: ~50% of plasma cholesterol, ~40% of 8-DHC, and<20% of 7-DHC were in the linoleate ester form. 8-DHC linoleate showed the strongest correlation with SLOS severity (r² = 0.68 without simvastatin). 7-ketocholesterol was one of three identified endogenous oxysterols and also correlated with severity (r² = 0.37). Antioxidants BHT and TPP effectively prevented ex vivo oxidation of 7-DHC during sample handling.
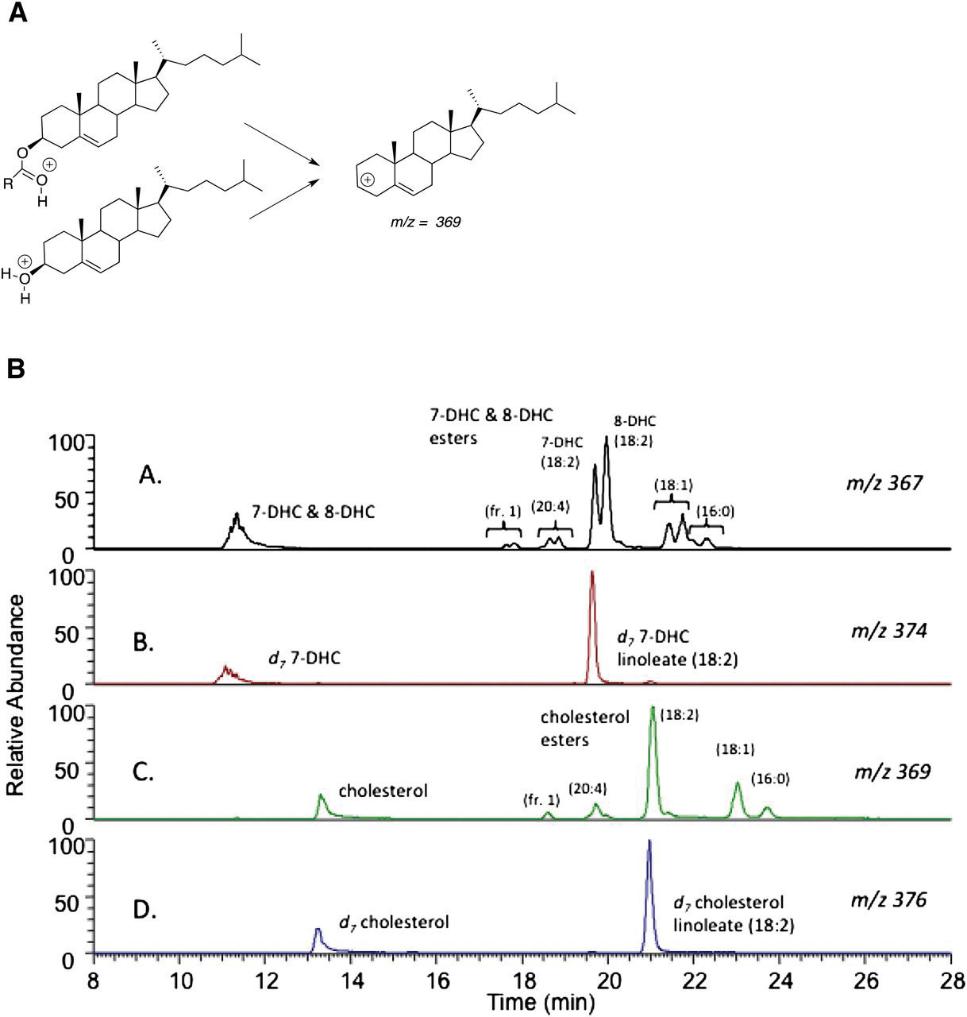 Free sterol and sterol ester analysis by LC-MS/MS.
Free sterol and sterol ester analysis by LC-MS/MS.
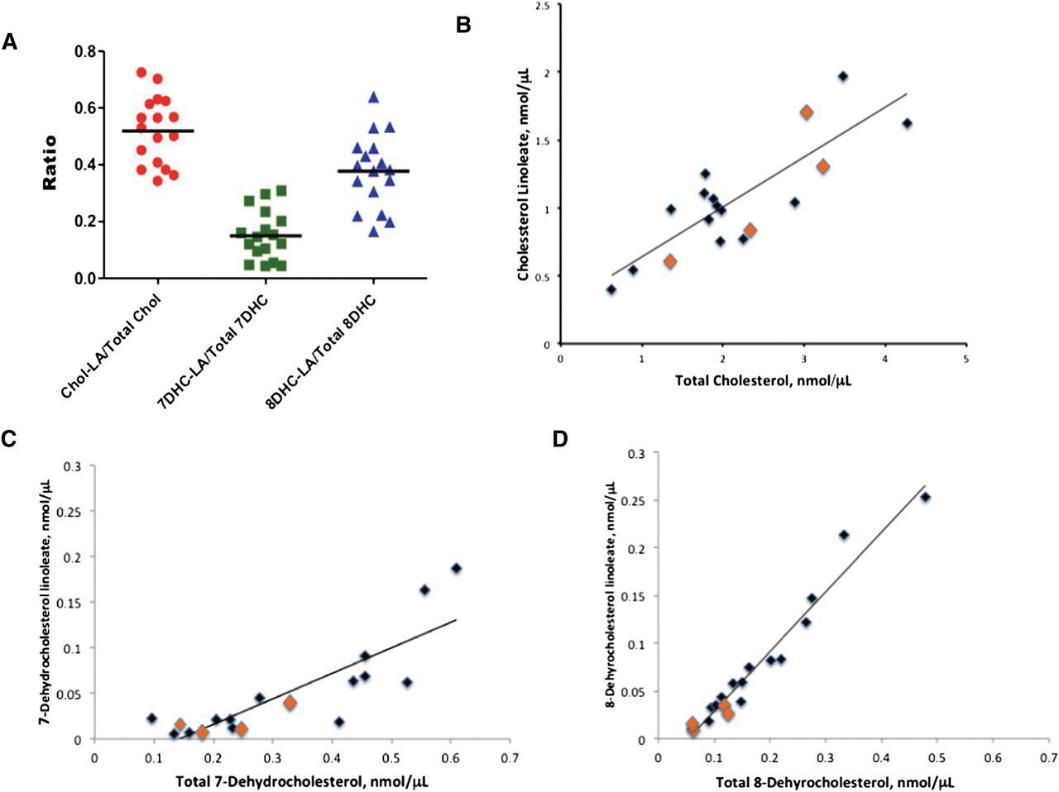 Linoleate ester:free sterol ratio determined for SLOS plasmas by LC-MS-MS
Linoleate ester:free sterol ratio determined for SLOS plasmas by LC-MS-MS
Conclusion
LC-MS/MS analysis of sterol esters in SLOS plasma identifies 8-DHC linoleate as a promising biomarker correlating with disease severity. Proper sample preservation is essential to prevent ex vivo oxidation, especially of 7-DHC.
Reference
- Liu, Wei, et al. "Assays of plasma dehydrocholesteryl esters and oxysterols from Smith-Lemli-Opitz syndrome patients [S]." Journal of lipid research 54.1 (2013): 244-253. https://doi.org/10.1194/jlr.M031732
Publications
Here are some publications in Metabolomics research from our clients:

- Prospective randomized, double-blind, placebo-controlled study of a standardized oral pomegranate extract on the gut microbiome and short-chain fatty acids. 2023. https://doi.org/10.3390/foods13010015
- Evidence for phosphate-dependent control of symbiont cell division in the model anemone Exaiptasia diaphana. 2024. https://doi.org/10.1128/mbio.01059-24
- The olfactory receptor Olfr78 promotes differentiation of enterochromaffin cells in the mouse colon. 2024. https://doi.org/10.1038/s44319-023-00013-5
- Multi‐omics identify xanthine as a pro‐survival metabolite for nematodes with mitochondrial dysfunction. 2019. https://doi.org/10.15252/embj.201899558
- Experimental microbial dysbiosis does not promote disease progression in SIV-infected macaques. 2018. https://doi.org/10.1038/s41591-018-0132-5