Why is Phosphoproteomics important?
Protein Modification
The transcription of DNA into mRNA requires translation into proteins with specific amino acid sequences to function in the body, and most of these proteins often require chemical modification to become active, which is called post-translational modification (PTM). Post-translational modification is the process of adding specific amino acids or changing specific chemical functional groups to the amino acid sequence of a protein, thereby changing the structure of the protein. More than three hundred potential PTM types have been experimentally demonstrated, and the same protein may be modified at multiple sites, contributing to the structural and functional diversity of proteins.
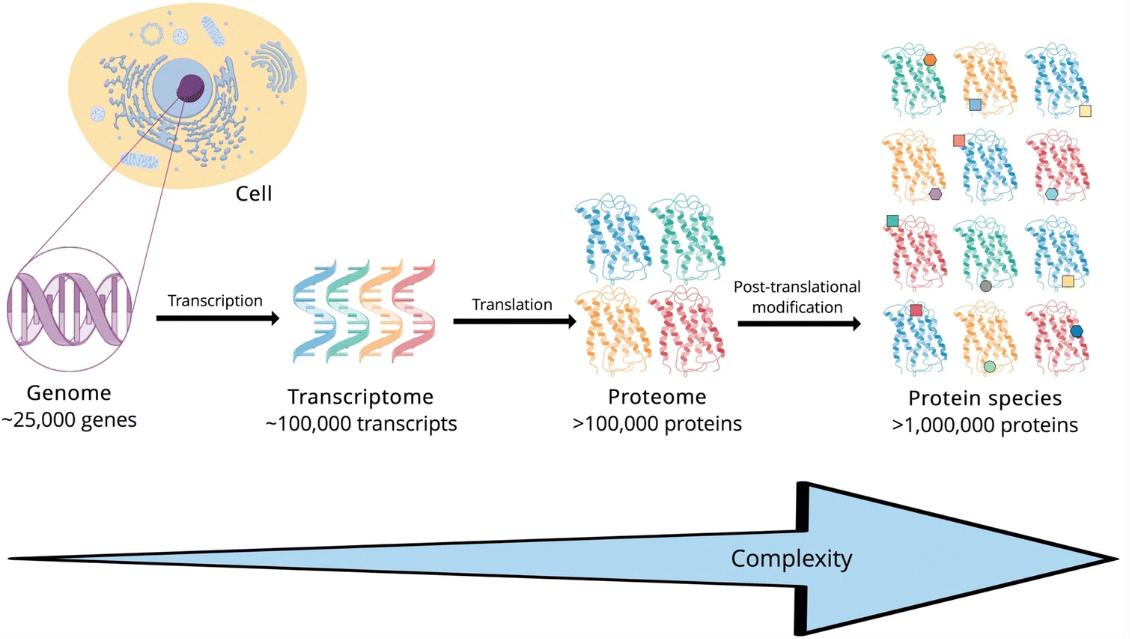
PTM increases the diversity of protein structure and function
What are Phosphorylation Modifications
Among the many PTM types, phosphorylation modifications account for about one-third of all proteins and are one of the most prevalent types of modifications. It affects intracellular signal transduction, cell structure, cell proliferation, apoptosis, transcription, metabolic processes, and regulation of the adaptive capacity of pathogenic microorganisms, etc. Therefore, the level of protein phosphorylation varies in different cells, and the phosphorylation level at specific sites may range from less than 1% to more than 90%.
The process of phosphorylation is to transfer the phosphate group of ATP to the amino acid side chain of protein under the catalysis of kinase, and then ATP becomes ADP. For most proteins, phosphorylation modification is a reversible transient modification. When a certain site of a protein helps the protein to complete its task, the protein will be dephosphorylated under the action of phosphatase, just like a "switch" for protein function, a small number of phosphorylations are permanent modifications.
Phosphorylation modifications can occur on a variety of amino acid residues and can be divided into four categories: 1. O-phosphorylation on hydroxyl residues of serine, threonine, tyrosine, and hydroxyproline; 2. Histidine N-phosphorylation on acid, lysine residues; 3. S-phosphorylation on cysteine residues; 4. Acyl-phosphorylation on aspartic acid, glutamic acid residues.
Phosphoproteomics Applications
Phosphoproteomics is a comprehensive analysis of phosphorylated proteins, including the identification, localization and quantification of phosphorylation.
Medicine:
Using mass spectrometry, more than 100,000 different phosphorylation events have been identified in human cells, which may affect the function of each protein. Many studies have shown that phosphorylation of some important biomarkers is dysregulated in diseases such as lung cancer, skin cancer, chronic myeloid leukemia, Alzheimer's disease, and diabetes[1][2]. For example, an article published in "Nature" in 2019 used PP to combine proteomics, transcriptomics, and whole genome sequencing to find new therapeutic targets for early-stage liver cancer[3].
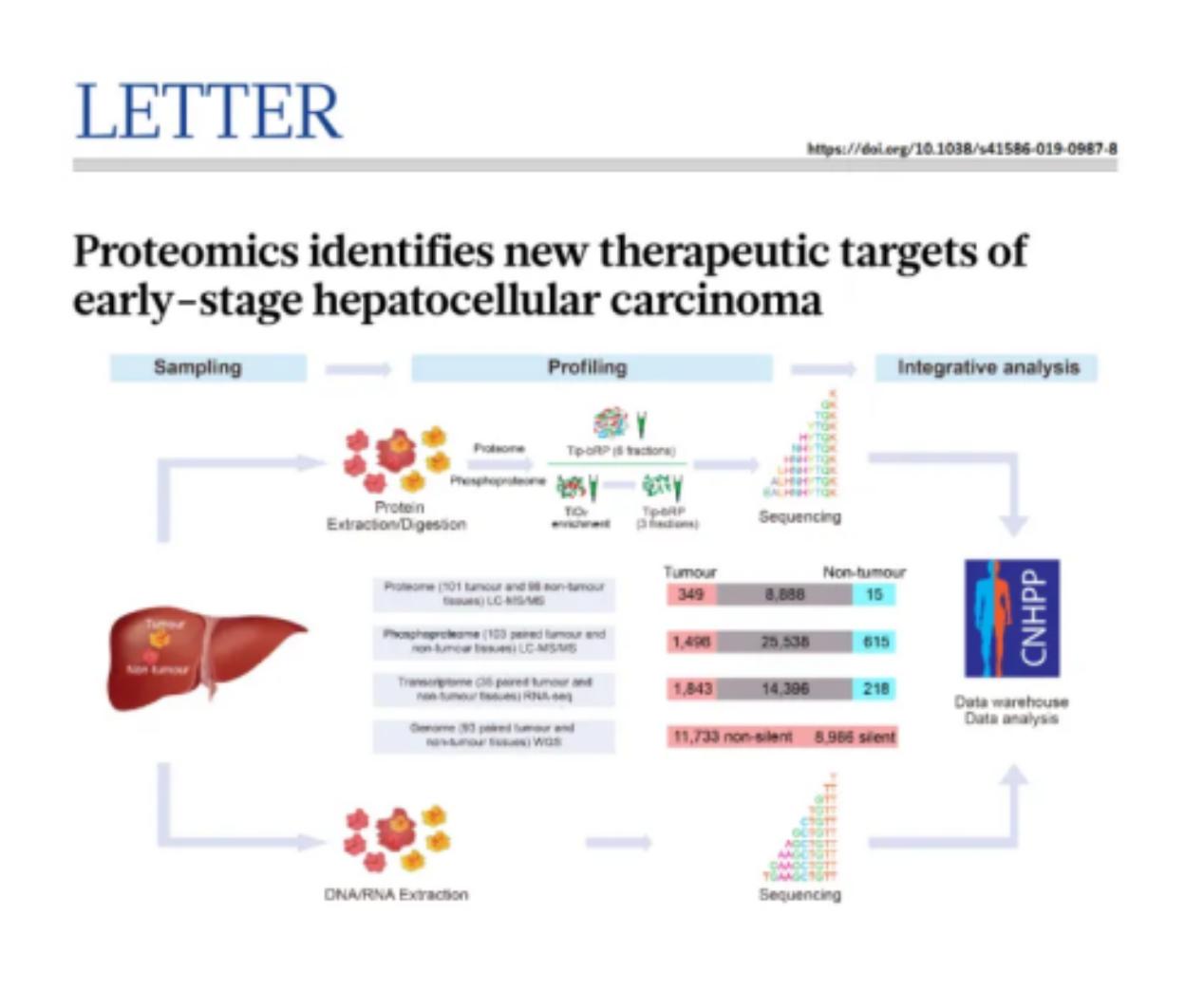
Application case of phosphorylated proteins in liver cancer research
Plants.
In plants, phosphorylation has been found to be involved in most metabolic and physiological processes such as temperature stress, salt stress, drought stress, and hormone regulation in plants. In January 2020, an article published in Nature used PP, proteomics and transcriptome to discover the presence of more than 18,000 proteins with more than 43,000 sites phosphorylated in more than 30 tissues of the model species Arabidopsis thaliana, further laying the foundation for the study of plant development and stress response, plant signaling and metabolic pathways, etc[4].
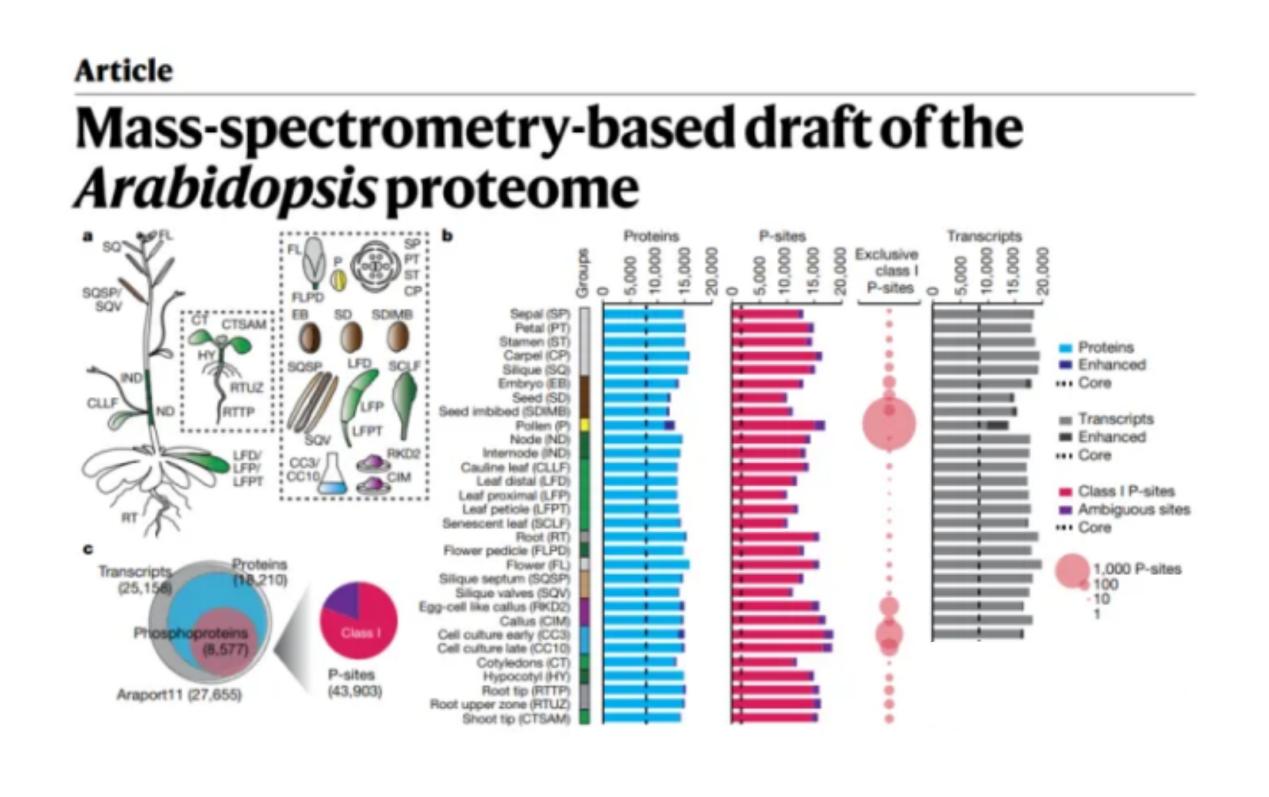
Figure 4 Examples of applications of phosphorylated proteins in plant research
Microorganisms.
In microbial studies, it has been found that PP affects the process of pathogen transmission, including adhesion to host cells and pathogen virulence, replication and persistence[5], so it has been extensively studied in the role of antibiotics as well as pathogen-host interactions.An article published in Molecular Plant in September 2020 reported that lectin receptor kinase in Arabidopsis thaliana would phosphorylate AvrPtoB, a pathogenic factor of Pseudomonas syringae, and reduce effector protein toxicity, thereby enhancing plant immunity[6].
Biological clocks.
Research related to biological rhythms has received more widespread attention due to the Nobel Prize in 2017, and the role of phosphorylation in this has gradually surfaced.In October 2019, an article published in Science found a large number of kinases present in synaptic proteins regulated by phosphorylation in a time-dependent manner and hypothesized that phosphorylation-mediated modulation of synaptic signaling may be a key driver of sleep and wake-dependent mechanisms that regulate synaptic homeostasis and function[7].
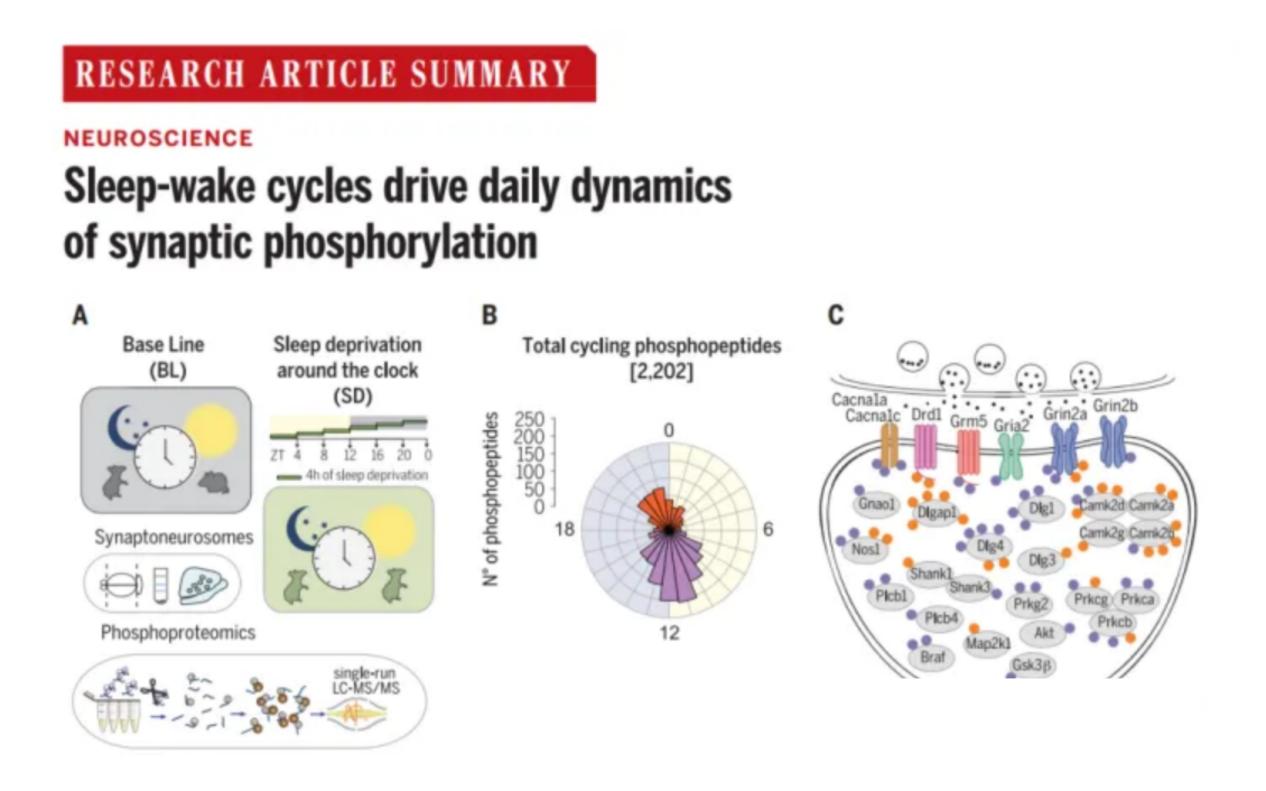
Application case of phosphorylated proteins in biological clock research
References
- Needham E J, Parker B L, Burykin T, et al. Illuminating the dark phosphoproteome[J]. Science signaling, 2019, 12(565).
- Harsha H C, Pandey A. Phosphoproteomics in cancer[J]. Molecular oncology, 2010, 4(6): 482-495.
- Jiang Y, Sun A, Zhao Y, et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma[J]. Nature, 2019, 567(7747): 257-261.
- Mergner J, Frejno M, List M, et al. Mass-spectrometry-based draft of the Arabidopsis proteome[J]. Nature, 2020, 579(7799): 409-414.
- Bonne Køhler J, Jers C, Senissar M, et al. Importance of protein Ser/Thr/Tyr phosphorylation for bacterial pathogenesis[J]. FEBS letters, 2020.
- Xu N, Luo X, Wu W, et al. A Plant Lectin Receptor-like Kinase Phosphorylates the Bacterial Effector AvrPtoB to Dampen Its Virulence in Arabidopsis[J]. Molecular Plant, 2020, 13(10): 1499-1512.
- Brüning F, Noya S B, Bange T, et al. Sleep-wake cycles drive daily dynamics of synaptic phosphorylation[J]. Science, 2019, 366(6462).
- Virág, D., Dalmadi-Kiss, B., Vékey, K. et al. Current Trends in the Analysis of Post-translational Modifications. Chromatographia 83, 1–10 (2020).






