Nitric oxide (NO) is an important regulator of a number of physiological processes such as vasodilation, inhibition of platelet aggregation, neurotransmission, and antimicrobial activity. Redox NO reacts with various proteins and regulates various intracellular and extracellular events. Apparently, NO modification reactions are carried out by transferring the nitroso group to the sulfhydryl group of cysteine to form S-nitrosothiols (SNO), a process commonly referred to as S-nitrosylation. It is well recognized that the regulation of S-nitrosylation occurring in proteins is similar to that of phosphorylation. Although all cysteine residues in a protein can undergo S-nitrosylation modifications, only few modifications are specific and serve to regulate protein function.
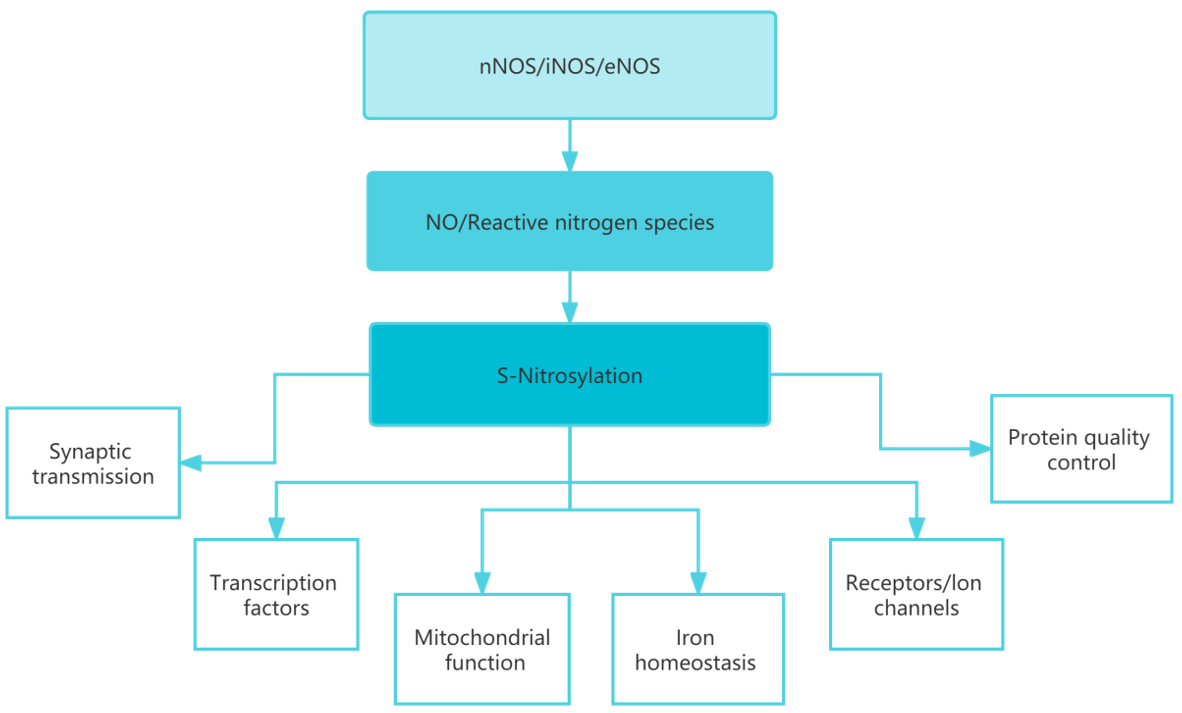
The most widely elucidated mechanisms of the NO signaling pathway include binding to and alteration of soluble guanylate cyclase, and cytochrome C oxidase. Protein S-nitrosylation is a reversible post-translational modification that is thought to be a regulator of protein activity, localization, protein stability and interactions between proteins.
Nitrosylation and denitrosylation
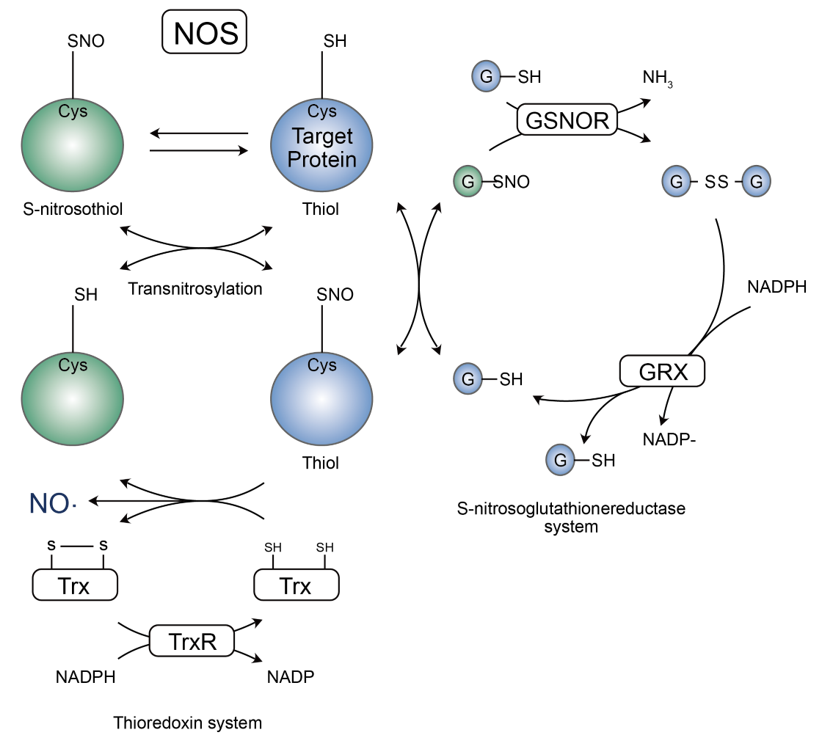
Thiol residues of proteins can specifically react with NO groups and different thiol substrates to form S-nitrosothiols, which can also be generated by the reaction of thiolates and S-nitrosoglutathione (GSNO). Due to the reversibility of this process, SNO also promotes the S-nitrosylation reaction, thus creating a dynamic equilibrium between redox GSH/GSNO and redox protein-SH/protein-SNO. In fact, GSNOR reacts with GSNO to generate oxidized glutathione disulfide (GSSG) and ammonia (NH3), thus reducing the concentration of S-nitroso alcohol. Subsequently, glutaredoxin (GRX) maintains and stabilizes this SNO system by reducing GSSG to GSH. Of course, NO molecules group can also be transferred directly to the protein to undergo modification, and the dynamic balance of S-nitrosothiols also relies on the thioredoxin/thioredoxin reductase (Trx/TrxR) system, where Trx-mediated reduction of SNO proteins to thiols is at the expense of oxidation of NAPDH, which ultimately and generates NO to mediate the relevant signaling pathway.
Nitrosylation and denitrosylation
Strategies for the identification of S-nitrosylated proteins
An increasing number of studies have focused on the systematic analysis of the degree of S-nitrosylation of proteins in tissues, organs and cells. To date, scientists have invested a great deal of work to understand the mechanisms of protein S-nitrosylation occurs and have explored several research tools to reveal protein S-nitrosylation. NO-based assays (Saville assay, chemiluminescence, colorimetric and fluorescence methods), direct capture based on organic mercury and hydrogenated scales, biotin conversion methods and liquid mass spectrometry are able to detect the degree of S-nitrosylation of proteins in cells and tissues under different physiological and pathological conditions.
Liquid mass spectrometry (LC-MS/MS) is an effective analytical tool that can qualitatively quantify SNO and SNO proteins in biological samples. However, direct discovery of SNO sites in organismal proteins is challenging because the S-NO chemical bond is very unstable. Compared to peptides without modifications, SNO-modified peptides usually have shifts to 29 Da molecular weight size.
Workflow
In-gel or in-solution digestion
Enrichment of modified peptides
HPLC separation followed by tandem mass spectrometry MS/MS analysis
S-nitrosylation data analysis






