Protein Post-translational Modification
Post-translational modification (PTM) plays an important role in the process of protein processing and maturation. It can change the physical and chemical properties of proteins, and affect the spatial conformation, steric hindrance and stability of proteins. In turn, it has an effect on the biological activity of the protein, causing a change in the function of the protein. Post-translational modified proteomics helps to reveal the molecular regulation mechanism of life activities, biomarker screening and drug target identification.
Post-translational Modification of Protein Drugs
Regulatory enzymes responsible for PTM of proteins have become the frontier and hot target in the field of new drug research. The integrity of protein drugs not only refers to the complete amino acid sequence, but also requires specific biological activities and functions produced after a series of protein PTM processes. The modification and processing methods of protein drugs have diverse characteristics, including but not limited to glycosylation, phosphorylation, ubiquitination, acetylation, methylation and so on. To this end, protein drug PTM analysis plays an important role in the development and production of protein drugs. At the same time, naturally modified protein drugs have an important role in determining their modification type, modification site, modification structure to improve their modification or processing efficiency, and can be used to improve the production process in the research and development or production process of biopharmaceutical companies, and eventually Improve the efficiency of biopharmaceutical development.
- Core histone post-translational modification
Post-translational modification of core histones is currently the most studied direction in drug research and development. Histone modification mainly includes acetylation, methylation, phosphorylation, ubiquitination, etc. These modifications can recruit recognition proteins that recognize the modification site, and then recruit other transcription factors or can form complexes with many physiological functions, and then carry out transcriptional regulation. Therefore, according to the mechanism of action of different regulatory factors, the researchers defined their image as "Writer", "Eraser", and "Reader". Write factors add modified enzymes to DNA or proteins. These modifications include methylation, acetylation, glycosylation, ubiquitination, phosphorylation, and oxidation (for proteins). The erasing factor is to remove the acetyl group on the histone lysine residue to change the charge and make the chromatin structure compact, inhibiting the transcriptional expression of the gene. Recognition factors can recognize these modified proteins. When these modifications are recognized by the recognition factors, they can recruit other transcription factors or proteins to jointly regulate physiological functions in the body.
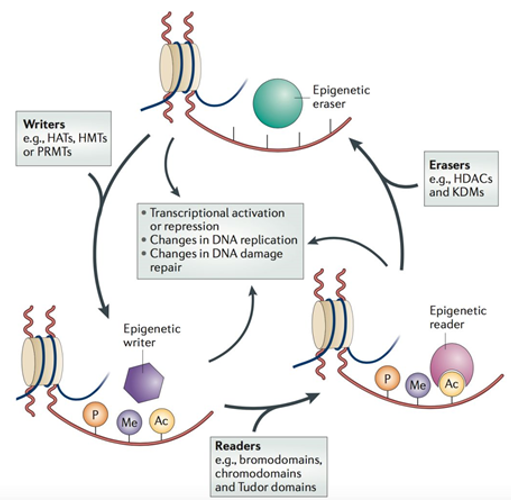
Histone translation and modification (Feinberg AP, 2018).
- Glycosylation of antibody drugs
Among the many PTMs of proteins, glycosylation modification is one of the most important and complex modifications, and one of the key quality attributes for evaluating antibodies. The realization of the monoclonal antibody drug function is closely related to its glycosylation modification, which will affect the performance of the protein, such as conformation, stability, solubility, pharmacokinetics, activity and immunogenicity. The most obvious role of protein glycosylation modification is that it can increase the stability and solubility of proteins. Different glycosylation modifications have different effects on the stability, half-life, safety, and biological activity of antibodies. For example, studies have shown that glycosylation can protect proteins by hiding their binding sites with proteases. Glycosylation modification can also hinder the binding of proteases to antibodies, thereby increasing the stability of antibodies.
Analysis Method of Protein Post-translational Modification
Mass spectrometry is an important method for identifying PTMs of proteins. Its principle is to use the mass shift of modified proteins to identify PTM sites. Biopharmaceutical products may contain a variety of variants or derivatives derived from functional PTMs or modifications, such as glycosylated variants (mAb) or ADCs. Therefore, we need a suitable technique for characterizing and quantifying substances with the above-mentioned complexity. Liquid chromatography-mass spectrometry (LC-MS) is a mature biopharmaceutical characterization technique because it can directly perform qualitative and quantitative determination at the molecular level. The application of this technology in other fields is also becoming more and more extensive, including macromolecular drug metabolism and pharmacokinetics (DMPK) research, process development, production, quality control, post-market supervision, and therapeutic drug monitoring.
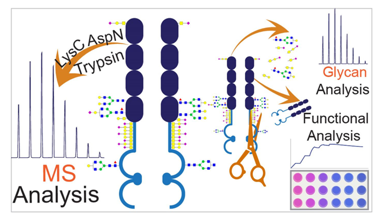
The workflow of Nano-LC-ESI-MS/MS analysis of permethylated Glycans (Fazel R et al. 2019).
- PTMScan®
PTMScan® is a proteomics technology specifically used for protein PTM research. It utilizes antibodies against protein PTM motifs to immunoaffinity enrich peptides with different PTM at the peptide level Peptides are quantified using liquid chromatography-tandem mass spectrometry (LC-MS / MS), enabling fast, accurate, and high-throughput analysis of key node protein phosphorylation, acetylation, methylation, and the changes of ubiquitination modification meet the needs of innovative research, biomarker identification, drug target screening and evaluation. PTMScan technology can identify new protein modification sites and open up new directions for protein function research, so it will be an important branch and trend of next-generation proteomics research.
References
- Feinberg AP. The Key Role of Epigenetics in Human Disease Prevention and Mitigation. N Engl J Med, 2018, 378, 1323-1334.
- Fazel R, Guan Y, et al. Structural and In Vitro Functional Comparability Analysis of Altebrel™, a Proposed Etanercept Biosimilar: Focus on Primary Sequence and Glycosylation. Pharmaceuticals, 2019, 12(1).





