Salvianolic Acid A Analysis Service
Creative Proteomics offers high-sensitivity salvianolic acid A analysis using LC-MS/MS and Orbitrap HRMS to support pharmacokinetic, metabolite profiling, and herbal standardization studies. We provide validated, multi-matrix testing solutions to help researchers accurately quantify salvianolic acid A, identify related metabolites, and ensure product consistency in both preclinical and botanical research.
Submit Your Request Now
×- What We Provide
- Advantage
- Workflow
- Technology Platform
- Sample Requirements
- FAQ
- Publication
What is Salvianolic Acid A?
Salvianolic acid A is a highly bioactive phenolic compound predominantly extracted from Salvia miltiorrhiza (Danshen), a traditional Chinese medicinal herb renowned for cardiovascular and hepatoprotective applications. Structurally, it belongs to the class of water-soluble caffeic acid derivatives and exhibits strong antioxidant, anti-inflammatory, anti-fibrotic, and neuroprotective effects.
With an impressive ability to scavenge reactive oxygen species and modulate critical cellular signaling pathways such as Nrf2/ARE and PI3K/Akt, salvianolic acid A has become a focal molecule in pharmacokinetic, pharmacodynamic, and metabolomic studies.
Salvianolic Acid A Analysis Service Offered by Creative Proteomics
- Targeted Quantification Analysis: Accurate measurement of salvianolic acid A in plasma, tissue, urine, bile, and herbal extracts using validated LC-MS/MS methods.
- Metabolite Identification and Profiling: Identification of primary and conjugated metabolites using Orbitrap-MS, supporting pathway elucidation.
- Pharmacokinetic Studies: Full PK profiling across species with time-course sampling and analysis of C_max, T_max, AUC, and half-life.
- Tissue Distribution: Determine compound localization in liver, brain, kidney, and other organs for bioavailability and targeting insights.
- Stability Testing: Evaluate compound integrity under various pH, temperature, light, and enzymatic conditions.
- Bioavailability Assessment: Compare oral and intravenous absorption using preclinical models.
- Microsomal Stability Assay: Assess in vitro metabolic stability via liver microsome incubation.
- Herbal Product Standardization: Ensure batch-to-batch consistency and regulatory compliance in Danshen extracts and formulations.
- Drug–Herb Interaction Screening: Analyze the effect of salvianolic acid A on CYP450 enzymes and transporters.
- Pathway Annotation: Map related metabolic pathways using KEGG and HMDB to support systems pharmacology research.
List of Detected Salvianolic Acid A and Related Metabolites
| Analyte | Classification | Associated Metabolic Pathway(s) |
|---|---|---|
| Salvianolic acid A | Polyphenolic antioxidant | Caffeic acid derivatives; Phenylpropanoid metabolism |
| Caffeic acid | Precursor compound | Phenylpropanoid biosynthesis |
| Rosmarinic acid | Intermediate metabolite | Tyrosine metabolism; Caffeic acid conjugation |
| Danshensu | Catabolic product | Hydroxycinnamic acid metabolism |
| Ferulic acid | Methylated phenolic acid | Methoxylated phenylpropanoid metabolism |
| Lithospermic acid B | Structural analog | Diterpenoid conjugates; Tanshinone co-pathways |
| Salvianolic acid B | Structural analog | Coumarin and polyphenolic biosynthesis |
| p-Coumaric acid | Phenolic precursor | Early phenylpropanoid pathway; cinnamic acid branchpoint |
Advantages of Salvianolic Acid A Assay
- Ultra-High Sensitivity: Detection limit (LOD) as low as 0.1 femtomole (fmol) using triple quadrupole LC-MS/MS, allowing accurate measurement of trace levels in complex matrices.
- Excellent Quantitative Precision: Relative standard deviation (RSD) of<5% across technical replicates; linearity (R²) of calibration curves typically exceeds 0.998 across the dynamic range.
- High Recovery Rate: Optimized extraction protocols yield ≥85% recovery of Salvianolic Acid A from plasma, serum, and plant matrices, minimizing sample loss.
- Wide Dynamic Range: Accurate quantification from 0.1 fmol to 1000 pmol, enabling simultaneous detection of basal and elevated metabolite concentrations.
- High Throughput Capacity: Capability to process 96–384 samples per batch, suitable for large-scale biomarker studies and population-based metabolomics.
- Cross-Matrix Compatibility: Validated workflows for multiple sample types, including human/animal biofluids, tissues, cell cultures, and plant extracts—with no loss in sensitivity or specificity.
- Data Traceability & Reproducibility: Use of internal standards and QC samples in every batch ensures analytical consistency across studies and platforms.
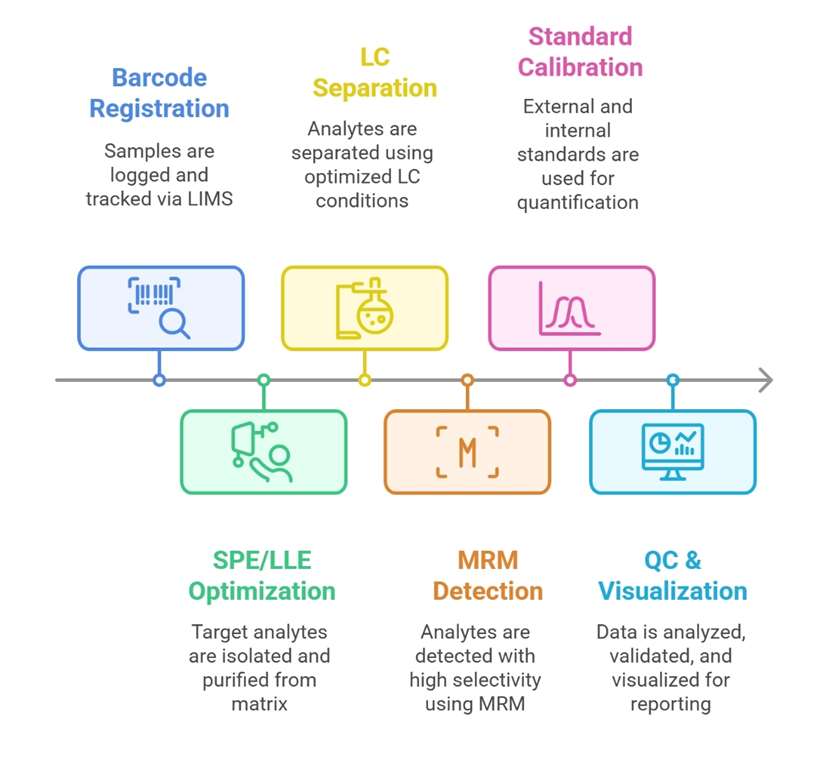
Workflow for Salvianolic Acid A Analysis Service
Technology Platform for Salvianolic Acid A Analysis Service
LC-MS/MS (SCIEX QTRAP® 6500+ with Waters UPLC): For targeted quantification in plasma, tissues, and herbal matrices
- LOD: 0.05 ng/mL
- Quantitative range: 0.05–1000 ng/mL
Orbitrap HRMS (Thermo Q Exactive™): For metabolite profiling and structural elucidation
- High-resolution, full-scan detection
- Supports untargeted pathway analysis
HPLC-DAD (Agilent 1260 Infinity): For routine detection in herbal extracts
- UV-based quantification at 286 nm
- Ideal for QC of botanical products

SCIEX Triple Quad™ 6500+ (Figure from Sciex)

Thermo Fisher Q Exactive (Figure from Thermo Fisher)

Agilent 1260 Infinity II HPLC (Fig from Agilent)
Sample Requirements for Salvianolic Acid A Analysis Service
| Sample Type | Minimum Amount Required | Notes |
|---|---|---|
| Plasma / Serum | ≥ 200 µL | Use EDTA or heparin tubes; avoid hemolysis |
| Whole Blood | ≥ 500 µL | Requires pre-processing to isolate nucleosides |
| Cell Pellet | ≥ 1 × 10⁷ cells | Washed with PBS; snap frozen or stored at −80°C |
| Animal Tissue | ≥ 50 mg | Flash-frozen preferred; homogenization required before extraction |
| Plant Tissue / Leaf | ≥ 100 mg | Fresh or flash-frozen; avoid degradation from light or heat |
| Microbial Culture Pellet | ≥ 50 mg wet weight / 1 × 10⁹ cells | Washed and frozen immediately post-centrifugation |
| Cell Culture Medium | ≥ 500 µL | Collect before media acidification; filter or centrifuge to remove cells |
| Urine | ≥ 1 mL | Midstream, fresh or frozen; no preservatives |
Applications of Salvianolic Acid A Assay Service
Pharmacokinetics
Assess absorption, distribution, metabolism, and excretion in preclinical models
Natural Product Research
Characterize active components in Salvia miltiorrhiza and related herbal formulations
Metabolomics
Explore metabolic fate and downstream pathway integration via targeted or untargeted approaches
Biological Research
Exploring the biological effects of salvianolic acid A in vitro and in vivo.
Toxicology
Assessing the safety profile of Sal A and its degradation products.
Herbal Standardization
Ensure consistency and content uniformity in botanical product batches
FAQ of Salvianolic Acid A Analysis Service
Can I submit multiple sample types in the same project (e.g., plasma and liver)?
Yes, we can process mixed sample types within a single project. Please ensure each type meets the respective volume and preservation requirements.
Do I need to provide reference standards or internal standards for analysis?
No, we provide high-purity salvianolic acid A reference standards and validated internal standards as part of our service.
How should I package and ship biological samples internationally?
We recommend shipping on dry ice using certified UN3373-compliant containers. Our support team can provide detailed packaging instructions upon request.
Can you help with data interpretation or pathway mapping?
Yes, we offer optional bioinformatics support, including metabolic pathway annotation using KEGG and HMDB databases.
How do you ensure sample traceability and data integrity?
All samples are barcoded upon arrival and tracked in our LIMS system with full audit trails from extraction to reporting.
Is it possible to run a small pilot study before committing to large-scale analysis?
Absolutely. We encourage pilot testing to optimize extraction conditions and confirm compound detectability in your sample matrix.
What file formats do you provide for raw and processed data?
We deliver results in Excel and PDF formats by default. Raw LC-MS/MS data files (e.g., .wiff, .raw) can be shared upon request.
Can you accommodate custom compound panels that include salvianolic acid A analogs?
Yes. We can customize your analysis panel to include structurally related compounds like salvianolic acid B, rosmarinic acid, or danshensu.
Learn about other Q&A.
Salvianolic Acid A Analysis Service Case Study

Title: Separation and identification of water-soluble salvianolic acids from Salvia miltiorrhiza Bunge by high-speed counter-current chromatography and ESI-MS analysis
Journal: Talanta
Published: 2006
- Background
- Materials & Methods
- Results
- Conclusion
- Reference
Salvia miltiorrhiza Bunge (Danshen) is a widely used traditional Chinese herb known for promoting blood circulation and treating blood stasis. Its active constituents include lipid-soluble tanshinones and water-soluble salvianolic acids. While tanshinones have been extensively studied, salvianolic acids, particularly salvianolic acid B, have recently garnered attention due to their pharmacological effects such as antioxidant activity, free radical scavenging, and neuroprotection. Efficient methods for isolating these compounds are needed for biochemical research, clinical applications, and quality control. High-speed counter-current chromatography (HSCCC), a liquid–liquid separation technique, is explored in this study as a preparative method to isolate and identify multiple salvianolic acids from Danshen.
Dried Salvia miltiorrhiza roots were sonicated in water and extracted twice. The extract was concentrated, purified using D101 macroporous resin, and freeze-dried to obtain crude salvianolic acids. Sample solutions (30–40 mg/mL) were prepared in the upper phase of the HSCCC solvent system.
The two-phase solvent system (n-hexane–ethyl acetate–water–methanol, 1.5:5:5:1.5, v/v) was selected based on optimal partition coefficients (K), measured by HPLC. The upper phase served as the stationary phase and the lower as the mobile phase.
HSCCC was carried out using a TBE-300A system with a 350 mL multi-layer coiled column at 850 rpm and 1.7 mL/min flow rate. Fractions were detected at 254 nm and collected manually.
Collected peaks were analyzed by HPLC (Agilent 1100) using a C18 column and isocratic elution (acetonitrile–water, 1:3 with 0.05% TFA) at 0.8 mL/min, monitored at 290 nm.
Peak identification was conducted via UV–vis spectrophotometry and ESI-MS (Agilent 1100 MSD) in full scan mode (m/z 100–1000), as well as LC–MS for structural confirmation.
Two solvent systems were screened for HSCCC separation of salvianolic acids. System 1 (n-hexane–ethyl acetate–water–methanol, 1.5:5:5:1.5) provided optimal partition coefficients and superior stationary phase retention. The optimized HSCCC conditions were: 25 °C, 1.7 mL/min flow rate, 850 rpm, and ≤ 80 mg sample load. Using these conditions, salvianolic acid B (peak IV) was isolated with a purity of 98.6% and yield of 63.4 mg (79.2%). LC–MS analysis identified peaks II–V as rosmarinic acid, lithospermic acid, salvianolic acid B, and likely salvianolic acid E, respectively.
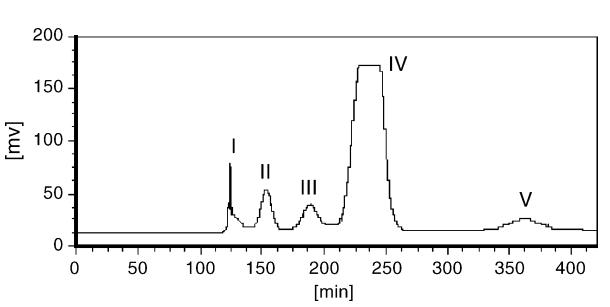 HSCCC chromatogram of crude salvianolic acids extracted from Salvia miltiorrhiza. Solvent system: hexane–ethyl acetate–water–methanol (1.5:5:5:1.5, v/v); stationary phase: upper; mobile phase: lower; flow rate: 1.7 mL/min; revolution speed: 850 rpm; temperature: 25 °C; sample: 80 mg in 2 mL upper phase; detection at 254 nm.
HSCCC chromatogram of crude salvianolic acids extracted from Salvia miltiorrhiza. Solvent system: hexane–ethyl acetate–water–methanol (1.5:5:5:1.5, v/v); stationary phase: upper; mobile phase: lower; flow rate: 1.7 mL/min; revolution speed: 850 rpm; temperature: 25 °C; sample: 80 mg in 2 mL upper phase; detection at 254 nm.
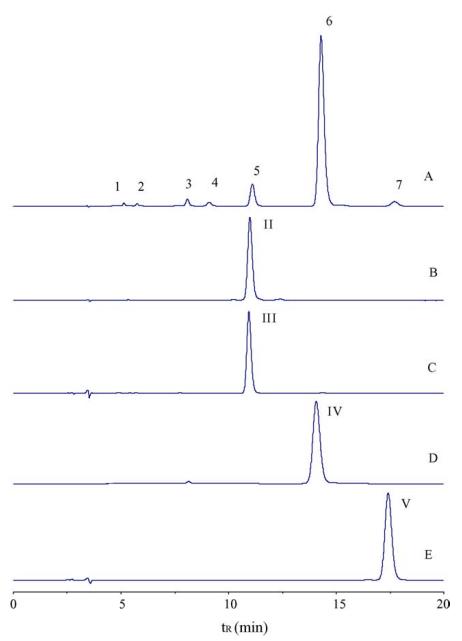 HPLC chromatograms of crude salvianolic acids (A) and purified peaks II–V (B–E). Column: Kromasil KR100-5C18 (150 × 4.6 mm); mobile phase: acetonitrile–water with 0.05% TFA; flow rate: 0.8 mL/min; detection at 290 nm.
HPLC chromatograms of crude salvianolic acids (A) and purified peaks II–V (B–E). Column: Kromasil KR100-5C18 (150 × 4.6 mm); mobile phase: acetonitrile–water with 0.05% TFA; flow rate: 0.8 mL/min; detection at 290 nm.
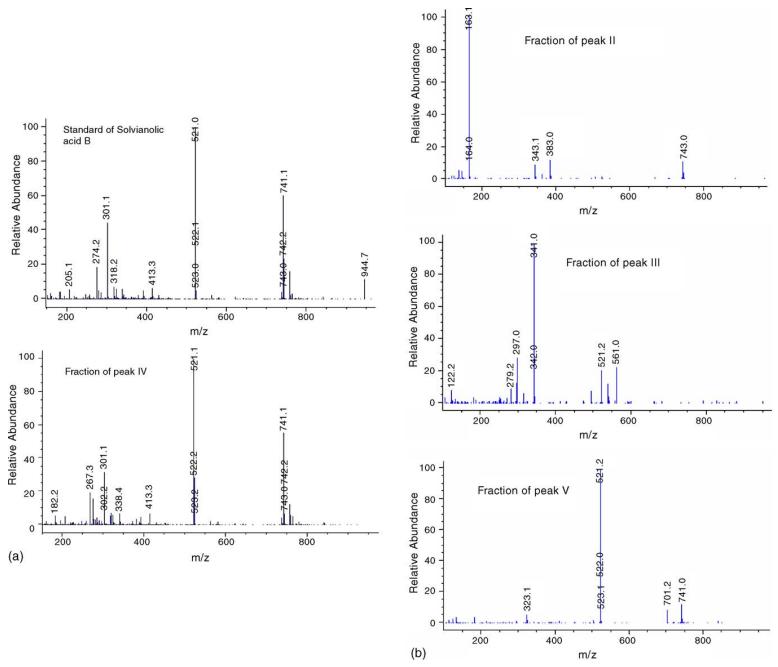 Mass spectra of salvianolic acid B standard and HSCCC-separated fractions: (A) standard and peak IV; (B) peaks II, III, and V.
Mass spectra of salvianolic acid B standard and HSCCC-separated fractions: (A) standard and peak IV; (B) peaks II, III, and V.
A preparative HSCCC method was successfully developed for the efficient separation and purification of salvianolic acids from Salvia miltiorrhiza. The optimized solvent system and operational parameters enabled high-purity isolation, especially of salvianolic acid B, demonstrating the method's reliability and scalability for natural product purification.
Reference
- Chen, Junhui, et al. "Separation and identification of water-soluble salvianolic acids from Salvia miltiorrhiza Bunge by high-speed counter-current chromatography and ESI-MS analysis." Talanta 69.1 (2006): 172-179. https://doi.org/10.1016/j.talanta.2005.09.041
Publications
Here are some publications in Metabolomics research from our clients:

- A human iPSC-derived hepatocyte screen identifies compounds that inhibit production of Apolipoprotein B. 2023. https://doi.org/10.1038/s42003-023-04739-9
- Characterization of CYCLOPHILLIN38 shows that a photosynthesis-derived systemic signal controls lateral root emergence. 2021. https://doi.org/10.1093/plphys/kiaa032
- Pan-lysyl oxidase inhibition disrupts fibroinflammatory tumor stroma, rendering cholangiocarcinoma susceptible to chemotherapy. 2024. https://doi.org/10.1097/HC9.0000000000000502
- Glycine supplementation can partially restore oxidative stress-associated glutathione deficiency in ageing cats. 2024. https://doi.org/10.1017/S0007114524000370
- Function and regulation of a steroidogenic CYP450 enzyme in the mitochondrion of Toxoplasma gondii. 2023. https://doi.org/10.1371/journal.ppat.1011566







