Electrospray ionization (ESI) is a technique to generate ions for mass spectrometry using electrospray by applying a high voltage to a liquid to produce an aerosol. Due to relatively fragile biomacromolecules, their structures are easily destroyed during the process of dissociation and ionization. ESI overcomes the tendency of these molecules to fragment upon ionization. ESI differs from other atmospheric pressure ionization processes, in which it may produce multiple charge ions, effectively extending the analyzer's mass range to accommodate the magnitude of kDa-mDa observed by proteins and their associated peptides.
The Principle of Electrospray Ionization
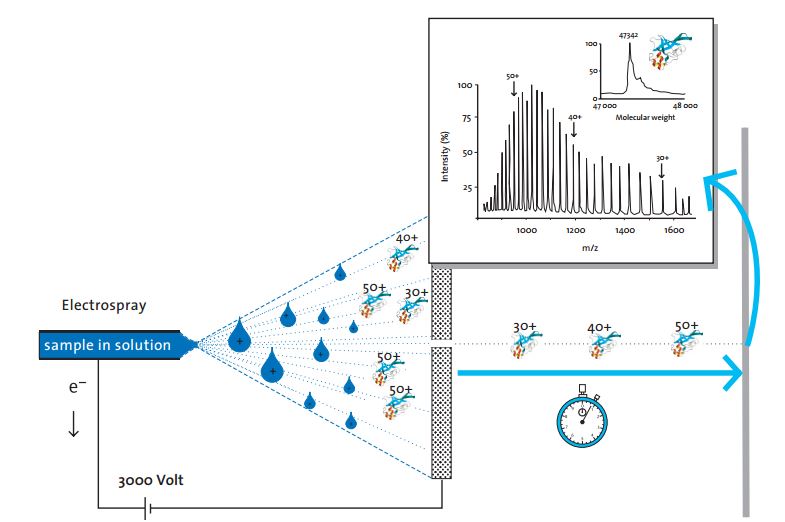
ESI applies a high voltage at the outlet of the capillary, and the high electric field generated atomizes the liquid flowing out of the capillary into tiny charged droplets. As the solvent evaporates, the charge intensity on the surface of the droplet gradually increases, and finally the droplet splits into one or a plurality of charged ions, allowing the analyte to enter the gas phase in the form of a single charge or multiple charges and become a gas phase ion.
There are two explanations for the mechanism of gas phase ion generation: the ion evaporation model (IEM) proposed by Thomsona and lribarne, and the charged residue model (CRM) advocated by Dole and Rllgen. In the two models, the ions to be analyzed are not excited by external energy and do not generate debris during the process of becoming a gas phase ion.
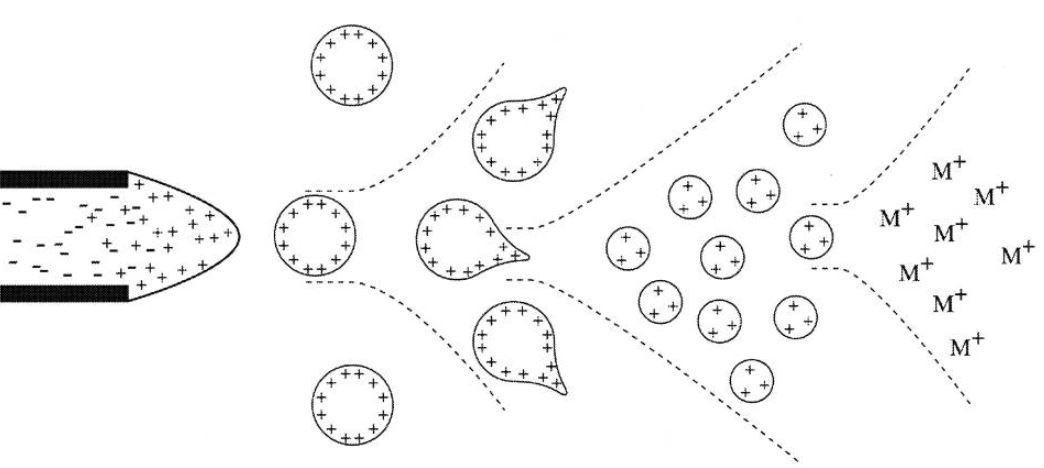
The Process of Electrospray Ionization
The ESI process can be roughly divided into three stages: droplet formation, desolvation, and gas phase ion formation.
- Droplet formation. Under the action of a high voltage electric field, the sample solution is ejected from the capillary to form a charged droplet.
- Desolvation. The droplets entering the spray chamber are evaporated by the countercurrent of the heated dry gas (like nitrogen gas), and the diameter of the droplets becomes smaller, and the surface charge density increases. When reaching the Rayleigh limit, the Coulomb repulsive force between the charges is sufficient to counteract the surface tension of the droplet, and the droplets fission, resulting in smaller charged droplets.
- Gas phase ion formation. The size of the charged droplets reaches the nm level, and the ions in the droplets turn into gas phase ions.
Advantages and Disadvantages of Electrospray Ionization
As a new type of soft ionization technology, ESI has been widely used in many fields. Although this technology makes up for shortcomings of some traditional mass spectrometry techniques, it still has some drawbacks.
- Advantages of ESI
- Electrospray provides a relatively simple way to ionize non-volatile solutions, allowing the mass spectrometer to provide a sensitive direct detection. Electrospray mass spectrometry can be used not only for the detection and analysis of inorganic substances, but also for the analysis of organometallic ion complexes and biomacromolecules.
- In electrospray mass spectrometry, high molecular weight molecules typically carry multiple charges, and the distribution of charge states accurately quantifies molecular weight, providing both accurate molecular mass and structural information.
- Multiple ionization modes to choose from: positive ion mode and negative ion mode.
- Effectively combined with a variety of chromatographs for complex system analysis.
- Disadvantages of ESI
- The experimental parameters or technical conditions must be carefully selected based on the problem to be solved.
- There is a limit to the choice of solvent and the range of solutions that can be used. At the same time, the response of the mass spectrometer to different complexes varies widely, which prevents accurate quantitative analysis.
- Since the solution parameters control the spray process, there is fluctuation in the ion signal even under good conditions.
Applications of ESI
ESI, a soft ionization technology, solves the problem of high polarity, ionization of thermally unstable proteins, and determination of the molecular mass of macromolecular organisms. Moreover, ESI has significantly improved sensitivity, accuracy, and complexity of analytical mixtures over previous mass spectrometry techniques, broadening the application of mass spectrometry in the protein field.
- Peptides and proteins. ESI can input the charge into the protein solution, then evaporate the solvent, and finally obtain the charged peptide. ESI-MS has the function of separation and identification, and is often used to identify complex peptide mixtures, such as mixed protein lysates or mixed protein bands on SDS-PAGE gels. ESI-MS is especially suitable for tandem mass spectrometry because ESI-MS can generate multiple charge peaks, while multi-charged ions are easily broken, which is an increase in collision activation sensitivity. It is used to monitor target peptides and obtain complete sequence information.
- Nucleotide. The emergence of ESI provides a powerful method for oligonucleotides and their similar structural and sequence analysis. ESI is to partially degrade the sample of the tested oligonucleotide, and sample it at a different time for mass spectrometry to obtain the molecular ion peak signal of partial degradation of the oligonucleotide. Comparing the molecular masses of two adjacent fragments, the molecular mass of the cleaved nucleotide monomer can be calculated, and the sequence of the oligonucleotide can be read by comparing it with the standard molecular weight of the four deoxynucleotides.
- The molecular mass of the ions. Electrospray ionization is characterized by the generation of highly charged ions rather than fragment ions, which reduces the mass-to-charge ratio (m/z) to a range that can be detected by most mass spectrometers, thus greatly expanding the molecular weight analysis range. The molecular mass of the ions can also be calculated from the mass-to-charge ratio and the number of charges.
ESI is a soft ionization method, which promotes the widespread use of the ESI source in mass spectrometry. Even compounds with large molecular weight and poor stability will not decompose during ionization.
ESI-MS Service
As a leading provider of ESI-MS technology, we offer a broad spectrum of analysis, catering not only to molecular weight determination and peptide map analysis but also the analysis of Host Cell Residual Proteins (HCPs).
Furthermore, our cutting-edge technology allows for the study of the protein folding path, analyzing the conformational changes of protein molecular ions, acquiring transition state information of conformational changes and unwinding the intricate process of spatial conformation.
Our ESI-MS technology also enables the study of the fascinating non-covalent impact of peptides and metals, as well as the non-covalent effects of protein and protein, peptide and peptide, protein and DNA, and protein and peptide. We do this by analyzing the intramolecular binding force or non-covalent binding selectivity, determining the affinity and stability, calculating binding constants or dissociation constants, and identifying the exact binding sites.
References
- Ho, CS; et al. Electrospray Ionisation Mass Spectrometry: Principles and Clinical Applications. Clin Biochem Rev. 2003,24 (1): 3–12.
- Pitt; James, J. Principles and Applications of Liquid Chromatography-Mass Spectrometry in Clinical Biochemistry. Clin Biochem Rev. 2009, 30 (1): 19–34.
- Markides, K; Gräslund, A. Mass spectrometry (MS) and nuclear magnetic resonance (NMR) applied to biological macromolecules. Advanced information on the Nobel Prize in Chemistry. 2002.
- Teo, CA; Donald, WA. Solution additives for supercharging proteins beyond the theoretical maximum proton-transfer limit in electrospray ionization mass spectrometry. Anal. Chem. 2014, 86 (9): 4455–62.
- Monge; et al. Mass Spectrometry: Recent Advances in Direct Open Air Surface Sampling/Ionization. Chemical Reviews. 2013, 113 (4): 2269–2308.
- Li, Anyin; et al. Synthesis and Catalytic Reactions of Nanoparticles formed by Electrospray Ionization of Coinage Metals. Angewandte Chemie International Edition. 2013, 53 (12): 3147–3150.








