Electron ionization (EI, formerly known as electron impact ionization and electron bombardment ionization) is the basis for one of the most efficient mass spectrometry methods for identifying a given organic compound. It is an ionization method in which high energy electrons interact with atoms or molecules in solid or gas phases to produce ions. This technique is considered to be a hard ionization method (high fragmentation) because it uses high-energy electrons to generate ions. This leads to extensive fragmentation, which contributes to the structural determination of unknown compounds. EI is useful for organic compounds with molecular weights below 600. In addition, several other thermally stable and volatile compounds in solid, liquid and gas states can be detected with the use of this technique when combined with various separation methods.
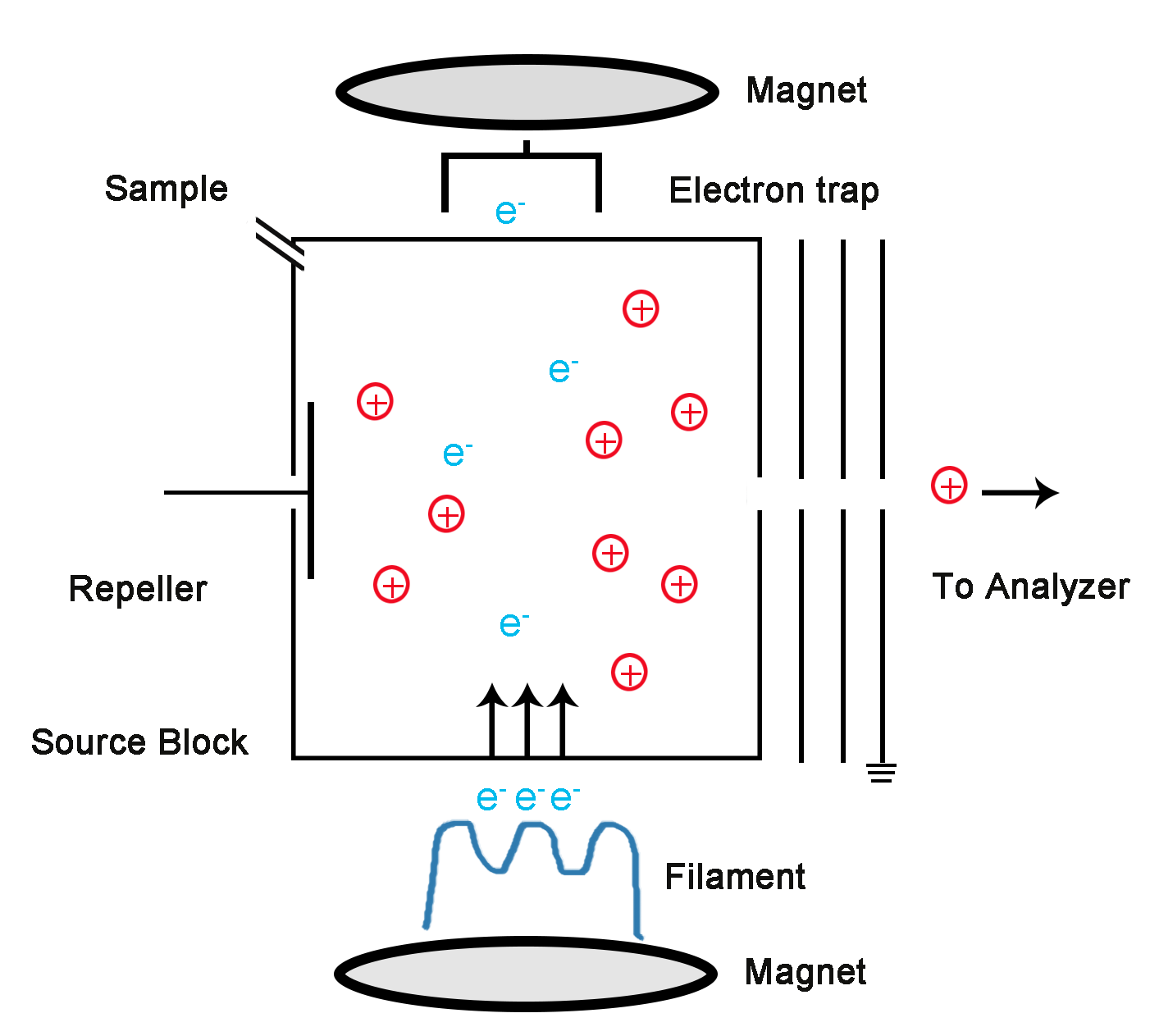 Figure 1. Schematic of electron ionization instrumentation
Figure 1. Schematic of electron ionization instrumentation
Principle of EI
In general, the ion source is to convert the sample into ions to accelerate and focus the ions into an ion beam that passes through the slit into the mass analyzer. The difference is obtained in the behavior of different ions in the electric or magnetic field, the mass spectrum is obtained by separating the ions by the mass-to-charge ratio, and the qualitative and quantitative results of the sample are obtained by analyzing the mass spectrum.
The EI source is generally composed of a cathode (filament), an ion chamber, an electron receiver, and a set of electrostatic lenses. Under high vacuum conditions, a current is applied to the filament to emit electrons, and electrons are accelerated from the filament to the electron receiving end. In this process, the sample molecules collide with electrons in the ion chamber, causing the sample molecules to ionize or fragment into fragments. In order to stabilize the generated ion current, the energy of the electron beam is generally set to 70 eV, which results in a stable standard mass spectrum.
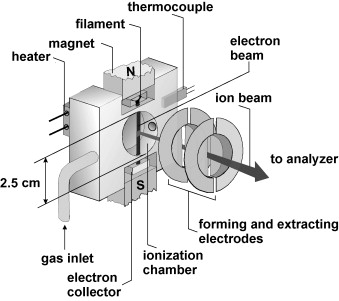 Figure 2. The electron impact ion source (Gluch K. et al., 2008)
Figure 2. The electron impact ion source (Gluch K. et al., 2008)
The gas or vapor being analyzed enters the ion source of the instrument and is converted to ions. The electrons are emitted by a direct-heating cathode (made of a multi-purpose wire), and a direct current voltage (70 V) is applied between the ionization chamber (positive electrode) and the cathode (negative electrode) to accelerate the electrons into the ionization chamber. When these electrons bombard atoms or molecules(M) in gas (or vapor) in the ionization chamber, the atoms or molecules(M) lose electrons into positive ions or molecular ions(M+):
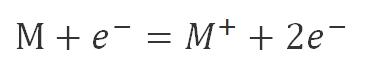
Molecular ions continue to be bombarded by electrons, making some chemical bonds to break, or causing rearrangements to break into multiple fragment ions or positive ions at an instantaneous rate.
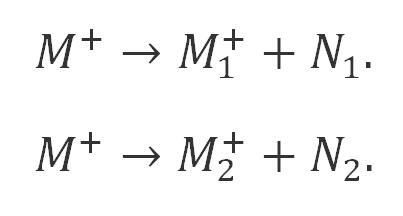
Advantages and Disadvantages of EI
The classic EI method has many advantages.
- EI is non-selective ionization and can be ionized as long as the sample can be vaporized.
- EI has high ionization efficiency and sensitivity.
- EI spectrum provides a wealth of structural information and is the “fingerprint” of the compound.
But there are some defects about EI.
- The EI source is not suitable for volatile, thermally unstable samples.
- Some compounds are fragile in EI mode and do not give an accurate mass spectra.
- The EI method can only detect positive ions and does not detect negative ions.
Applications of EI
EI, one of the earliest ion sources for mass spectrometry, is widely used in instruments. The GC - MS is usually associated with an EI, which can be used to analyze small molecules, volatile, thermal stability, and vaporizing compounds. Currently, the GC-EI-MS can be incorporated for the analysis of biological fluids, elemental of organic species, pesticide residue and others.
- Analysis of Biological Fluids. GC-EI-MS can be used to analyze biological fluids in a variety of applications. For example, GC-EI-MS is used in protein turnover research, which measures a very low level of d-phenylalanine, indicating the enrichment of amino acid incorporated into tissue protein during studies of human protein synthesis.
- Elemental Analysis of Organic Species. EI can be also used for the elemental analysis of organic species, and the instrument of electron ionization high-resolution mass spectrometry (EI-HRMS) can be used to elemental analysis (EA) of organic molecules (rather than just individual ions). EI-HRMS determines the type of organic elements by measuring the proportion of elements with total organic mass, such as oxygen/carbon (O/C), hydrogen/carbon (H/C), nitrogen/carbon (N/C), and the organic mass to organic carbon ratio (OM/OC).
- Pesticide Residue Analysis. GC-EI-MS has been successfully applied to determine the pesticide residues in fresh food through a single injection analysis. This technology identifies 81 multi-class pesticide residues in vegetables.
In conclusion, advantages based on various operations of EI have been widely used in various industries. EI is mainly used in combination with GC-MS for environmental analysis, qualitative and quantitative analysis of drugs and metabolites in biological samples, and the determination of unknown components.
We have briefly introduced the EI, a type of ionization method, which can help you understand more about mass spectrometry. At Creative Proteomics, we have developed the professional mass spectrometry platform that contains state-of-the-art instruments. By using mass spectrometry, Creative Proteomics can provide different services to meet various requirements, including:
References
- Kaufman, H. R. Performance correlation for electron-bombardment ion sources. 1965.
- Mcnaught, A. D.; Wilkinson, A. Iupac compendium of chemical terminology. Encyclopedic Dictionary of Polymers. 2006.
- Dass, C. Fundamentals of contemporary mass spectrometry. John Wiley & Sons. 2007.
- Watson, J. T.; et al. Electron Ionization Mass Spectrometry. Encyclopedia of Analytical Chemistry. John Wiley & Sons, 2006:1275-1278 vol.2.
- Głuch, K.; et al. Electron impact ionization of acrylonitrile. International Journal of Mass Spectrometry. 2008:278(1), 10-14.
- Cappiello, A.; et al. A simple approach for coupling liquid chromatography and electron ionization mass spectrometry. Journal of the American Society for Mass Spectrometry. 2002: 13(3), 265.
- Eustergerling, B. Application of laser-induced electron impact ionization to the deposition chemistry in the hot-wire chemical vapor deposition process with SiN 4 -NH 3, gas mixtures. Journal of the American Society for Mass Spectrometry. 2007:18 (11), 1950-1958.
- Cappiello, A.; et al. New trends in the application of electron ionization to liquid chromatography-mass spectrometry interfacing. Mass Spectrometry Reviews. 2010: 20(2), 88-104.
- Mol, H. G.; et al. Evaluation of gas chromatography - electron ionization - full scan high resolution Orbitrap mass spectrometry for pesticide residue analysis. Analytica Chimica Acta. 2016, 935:161-172.




