Hydroxycholesterols Analysis Service
Hydroxycholesterol analysis you can trust—quantify oxysterols with LC–MS/MS at low-ng/mL, isomer-resolved, and artifact-controlled.
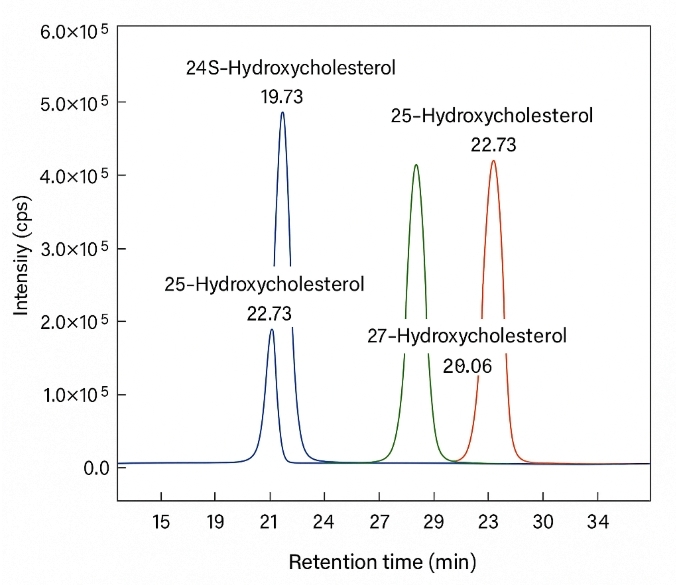
- Isomer-level resolution for 24S-, 25-, 27-, 7α-/7β-, 4β-HC, 7-keto, epoxides
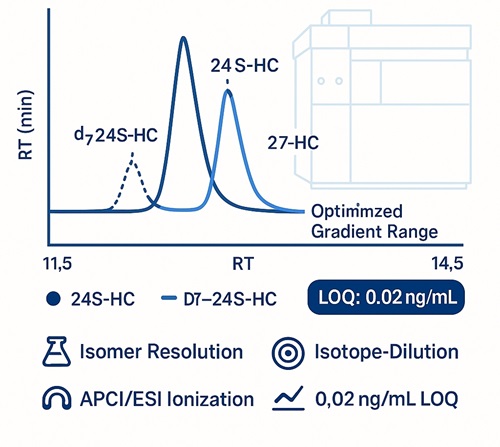
- LOQ as low as 0.02 ng/mL via isotope-dilution LC–MS/MS
- Full workflow control: sample prep, internal standards, gradient tuning
- Validate oxidation markers in plasma, CSF, tissues, foods, formulations
Submit Your Request Now
×
What You Will Receive
- Raw data files: .raw, .wiff, and .mzML
- Quant tables with internal standard normalization
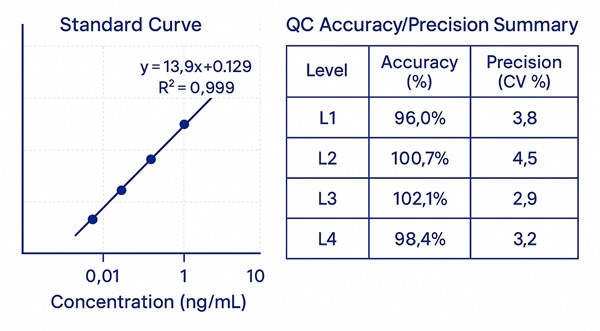
- Calibration curves, QC performance summaries
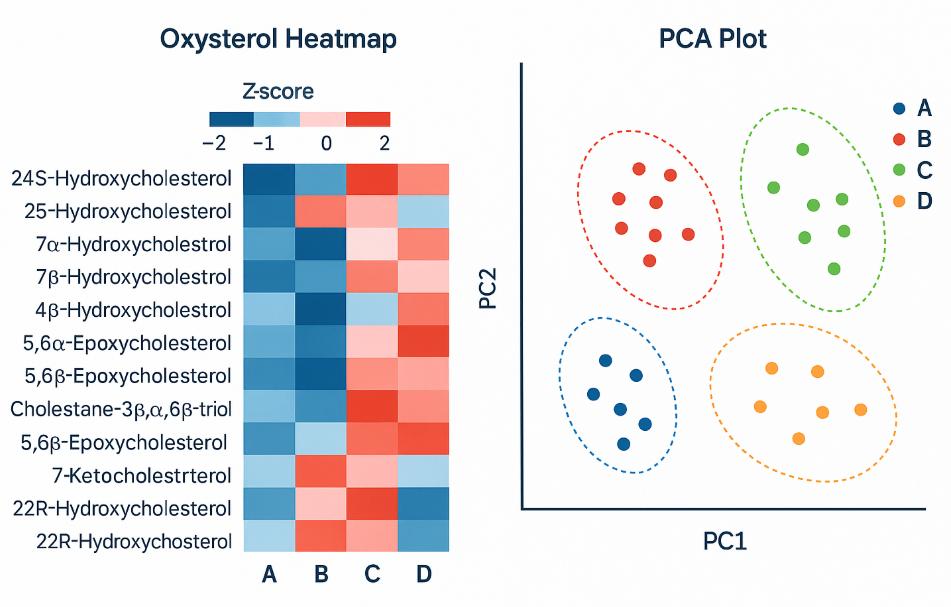
- Chromatograms (XIC/TIC) and PCA/heatmap visuals
- Annotated method file: MRM transitions, RTs, surrogates
- What We Provide
- Advantages
- Technology Platform
- Sample Requirement
- Demo
- FAQs
What Are Hydroxycholesterols?
Hydroxycholesterols—often referred to as oxysterols—are oxidized derivatives of cholesterol formed enzymatically (e.g., 24S-, 25-, 27-hydroxycholesterol) or non-enzymatically (e.g., 7-ketocholesterol, epoxides). They influence membrane organization, bile acid and steroid biosynthesis, and ligand activity at nuclear receptors. In R&D, oxysterols are sensitive readouts for cholesterol turnover, CYP pathway interrogation, formulation stability, food/nutrition research, and cellular stress evaluation. Accurate quantification is technically demanding due to low abundance, isomeric complexity, and artifactual oxidation—precisely the challenges our platform addresses.
Hydroxycholesterol Analysis Services Offered by Creative Proteomics
- Targeted oxysterol quantification (LC–MS/MS, isotope dilution).
- Expanded oxysterol library screening (HRAM LC–MS).
- GC–MS/MS of TMS-derivatized oxysterols for orthogonal confirmation.
- Isomer-resolved methods (derivatization and gradient tuning for critical pairs).
- Total vs. free oxysterols (selective hydrolysis for esterified pools).
- Stable-isotope tracing (e.g., [2H] cholesterol incorporation to track flux).
- Formulation/food stability studies with oxidation-product profiling.
Full List of Detectable Hydroxycholesterols and Related Lipids
| Class | Analyte (Common) | Synonym/Note | Typical LOQ (ng/mL) | Internal Standard (example) |
|---|---|---|---|---|
| Primary hydroxycholesterols | 24S-Hydroxycholesterol | CYP46A1 product | 0.02–0.05 | d7-24S-HC |
| 25-Hydroxycholesterol | CH25H product | 0.02–0.05 | d6-25-HC | |
| 27-Hydroxycholesterol | 26R-HC (CYP27A1) | 0.05–0.10 | d6-27-HC | |
| 7α-Hydroxycholesterol | Bile acid pathway | 0.05–0.10 | d7-7α-HC | |
| 7β-Hydroxycholesterol | Non-enzymatic/epimer | 0.05–0.10 | d7-7β-HC | |
| Regulatory/biomarker | 4β-Hydroxycholesterol | Endogenous CYP3A readout | 0.05–0.10 | d7-4β-HC |
| Epoxides & triols | 5,6α-Epoxycholesterol | 0.10–0.20 | d7-5,6-Epoxy | |
| 5,6β-Epoxycholesterol | 0.10–0.20 | d7-5,6-Epoxy | ||
| Cholestane-3β,5α,6β-triol | From epoxide hydrolysis | 0.10–0.20 | d7-Triol | |
| Keto-oxysterol | 7-Ketocholesterol | Oxidation marker | 0.05–0.10 | d7-7-Keto |
| Additional targets | 22R-Hydroxycholesterol | 0.05–0.10 | d7-22R-HC | |
| 20S-Hydroxycholesterol | 0.10–0.20 | d6-20S-HC | ||
| 24,25-Epoxycholesterol | 0.10–0.20 | d6-24,25-Epoxy |
Advantages of Our Hydroxycholesterols Analysis Services
- Sensitivity: LOQs down to 0.02 ng/mL for key oxysterols with charge-tag derivatization.
- Linearity: r² ≥ 0.995 across wide dynamic ranges (panel-specific).
- Precision: intra-batch %CV ≤ 10%, inter-batch %CV ≤ 15% under routine conditions.
- Recovery: 85–110% across validated matrices using matrix-matched calibration and isotopic surrogates.
- Carryover control: post-calibration blank signals < 0.1% of high standard peak area.
- Isomer separation: chromatographic resolution Rs ≥ 1.5 for 7α/7β and 5,6α/5,6β pairs on tuned gradients.
- Artifact minimization: spike-recovery stress tests show ≤ 5% artifactual 7-ketocholesterol formation with our workflow.
- Data integrity: S/N ≥ 10 at LOQ and mandatory qualifier/quantifier ion ratios within acceptance windows.
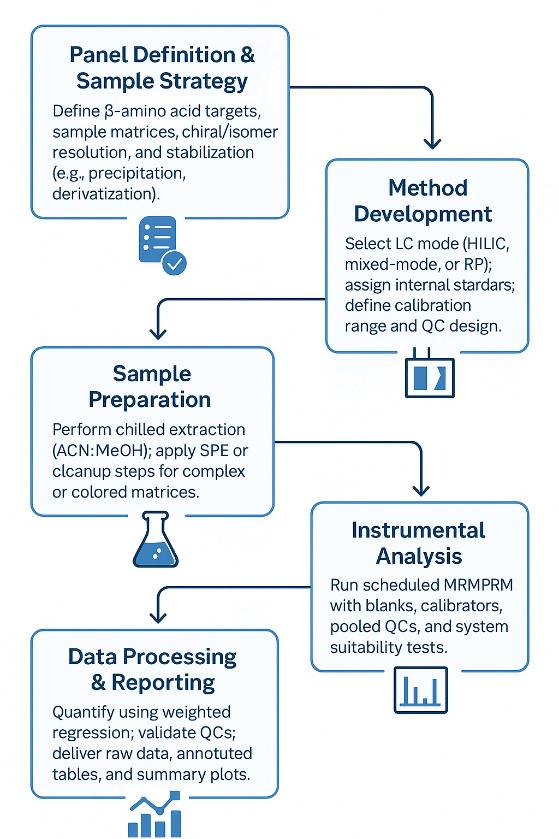
Workflow for Hydroxycholesterols Analysis Service
1. Consult & Define Objectives — Matrix, target panel, quant type (absolute/relative), expected ranges.
2. Method Selection & Quotation — Choose APCI-MRM / HRAM / SFC and QC depth; finalize target list and calibration levels.
3. Sample Intake & Pre-QC — Receipt check, storage, and small-scale extraction test for recovery and matrix effects.
4. Extraction & ISTD Spiking — MTBE or hexane-based CE-preserving extraction with BHT; add deuterated CE ISTDs.
5. Chromatography & MS Acquisition — Gradient LC per above; scheduled MRM or HRAM PRM; pooled-QC every 10 samples.
6. Data Processing & QC Review — Calibration, RT/ion-ratio checks, blank/carryover review, drift correction if needed.
7. Reporting & Handover — Raw files, annotated tables, QC report, figures (TIC/XIC, calibration plots, heatmaps/PCA), and interpretation highlights.
Technology Platform for Hydroxycholesterols Analysis Service
Creative Proteomics employs high-sensitivity LC–MS/MS and GC–MS platforms to enable precise, isomer-resolved hydroxycholesterol quantification across diverse biological and lipid-rich samples.
LC–MS/MS
High-sensitivity, targeted quantification using isotope-dilution MRM mode.
Instruments: Agilent 6495C
Ionization: APCI(+) for native oxysterols; ESI(+) after derivatization
Resolution of isomers: Optimized gradients for 7α/7β, 24S/25/27-hydroxycholesterols
Sensitivity: LOQ down to 0.02 ng/mL
HRAM LC–MS
Instrument: Thermo Q Exactive / Exploris 240.
Resolution: ≥ 70,000 (m/z 200); mass accuracy ≤ 3 ppm; PRM for isomer-specific fragments.
GC–MS
Used for orthogonal confirmation of derivatized oxysterols (e.g., TMS-derivatized epoxides, triols, and ketones).
Instruments: Agilent 7890B-5977A
Ionization: EI (70 eV) with SIM mode for enhanced selectivity
Note: Method optimization (e.g., derivatization, artifact control) is critical due to single-quadrupole configuration

Agilent 6495C Triple Quadrupole (Figure from Agilent)

Thermo Fisher Q Exactive (Figure from Thermo Fisher)

Agilent 7890B-5977A (Figure from Agilent)
Sample Requirements for Hydroxycholesterols Analysis Service
| Matrix | Recommended Amount | Container | Storage | Notes |
|---|---|---|---|---|
| Plasma/Serum | 100–200 μL | Amber polypropylene or glass | −80 °C | Avoid hemolysis; no freeze–thaw cycles |
| CSF | 200–500 μL | Low-bind tubes, amber if possible | −80 °C | Low lipid matrix; high sensitivity method applied |
| Tissue (animal/plant) | 20–100 mg | Pre-weighed cryovials | −80 °C | Record wet weight; keep frozen/low-light |
| Cell pellets | ≥1×10⁶ cells | Low-bind tubes | −80 °C | Wash with cold PBS; remove residual media |
| Culture media/bioreactor broth | 0.5–1.0 mL | Amber tubes | −80 °C | Filter particulates if feasible |
| Food/formulation oils & powders | 100–500 mg or μL | Amber glass vials | −20 °C to −80 °C | Provide matrix composition if available |
Demo Results
When Do You Need Oxysterol Quantification?
Cholesterol Turnover and Brain Research
Measure 24S-hydroxycholesterol as a direct readout of neuronal cholesterol elimination, or track 27-hydroxycholesterol for systemic cholesterol balance studies.
CYP Enzyme Activity and Drug Metabolism
Use 4β-hydroxycholesterol as an endogenous biomarker of CYP3A activity, or monitor 25-hydroxycholesterol to evaluate CH25H function in immune and metabolic models.
Formulation and Food Stability Testing
Detect early-stage oxidation products such as 7-ketocholesterol or 5,6-epoxides in oils, supplements, or liposomal formulations, ensuring product stability under stress conditions.
Oxidative Stress and Cellular Lipidomics
Profile oxysterols in cultured cells or tissues to evaluate lipid peroxidation, stress signaling, or membrane remodeling under different treatments.
Metabolic Flux Tracing
Incorporate stable isotope-labeled cholesterol to track oxysterol biosynthesis and pathway dynamics across biological systems.
FAQ of Hydroxycholesterols Analysis Service
How do you control for artifact formation during sample handling?
All samples are extracted under antioxidant- and chelator-protected conditions (e.g., BHT, EDTA/DTPA), with strict light control and temperature monitoring. Method blanks, spike-recovery tests, and degradation checks are performed to assess potential 7-ketocholesterol or epoxide artifacts.
Do you separate structurally similar isomers like 7α-/7β- or 24S-/25-hydroxycholesterol?
Yes. Our LC–MS/MS and HRAM methods are specifically tuned with derivatization and gradient optimization to resolve key oxysterol isomers. Resolution factors (Rs ≥ 1.5) are achieved for critical analytical pairs.
Can I quantify both free and esterified hydroxycholesterols?
Yes. We offer differential workflows for free oxysterols, or total oxysterols via selective hydrolysis. You may request either or both depending on your project goals (e.g., formulation stability, metabolic flux).
Are the methods suitable for quantifying trace levels in low-lipid matrices like CSF or cells?
Absolutely. Our charge-tag LC–MS/MS platform achieves LOQs down to 0.02 ng/mL for selected oxysterols. The methods are routinely used for CSF, low-yield cell lines, and brain tissue profiling.
Do you provide method files and raw data for downstream analysis?
Yes. All projects include full data handover: raw files (.wiff/.raw/.mzML), annotated quant tables, QC reports, chromatograms (TIC/XIC), and method metadata (calibration curve, MRM/PRM transitions, retention time).
Can stable isotope tracing be incorporated into hydroxycholesterol studies?
Yes. We support [²H]- or [¹³C]-cholesterol tracing for dynamic flux analysis. Target panels can be customized to detect labeled oxysterols and differentiate endogenous vs. exogenous contributions.
Do you provide comparative quantification across multiple batches or conditions?
Yes. Bracketed pooled-QC and internal normalization are applied across runs to ensure data comparability for relative or semi-quantitative interpretation.
How do your methods align with regulatory or publication standards?
All methods are supported with validation summaries (LOD/LOQ, linearity, precision, recovery). Reporting format aligns with journal expectations (e.g., lipidomics, oxidative stress studies) and regulatory-style bioanalytical guidance when requested.
Can you measure oxysterol profiles in combination with other lipid classes (e.g., cholesteryl esters, bile acids, sphingolipids)?
Yes. Integrated lipid panels are available upon request. We can co-profile oxysterols with neutral sterol esters, bile acid intermediates, and glycosphingolipids using matched extraction and MS workflows.
Can your method distinguish between enzymatic vs. non-enzymatic oxysterol formation?
Yes. By combining specific target panels (e.g., 24S-, 25-, 27-hydroxycholesterols for enzymatic; 7-Keto, 7β, 5,6-epoxides for oxidative stress), and by analyzing total vs. free oxysterols, we can help interpret the likely biosynthetic origin based on profiles and ratios.
Learn about other Q&A about proteomics technology.
Publications
Here are some of the lipidomics-related papers published by our clients:

- APOB100 transgenic mice exemplify how the systemic and retinal cholesterol metabolism are interconnected. 2024.
- A non-probiotic fermented soy product reduces cholesterol and improves lipid metabolism in humans. 2021.
- White matter lipid alterations during aging in the rhesus monkey brain. 2024.
- Characterization of Dnajc12 knockout mice, a model of hypodopaminergia. 2024.
- Annexin A2 modulates phospholipid membrane composition upstream of Arp2 to control angiogenic sprout initiation. 2023.