High-Purity Exosome Isolation Service with Multiple Workflow Options
At Creative Proteomics, we offer an exosome isolation service powered by ultra-high-speed centrifuges and validated protocols to ensure reliable yield and quality. Our platform supports a broad range of sample types, including:
- Cell culture supernatant
- Serum and plasma
- Urine
- Cerebrospinal fluid
The resulting exosomes are compatible with downstream techniques such as nanoparticle tracking analysis (NTA), transmission electron microscopy (TEM), and Western blotting (WB), supporting high-resolution exosome proteomic, exosome lipidomics, genomic, and functional investigations.
Submit Your Request Now
×
- Service Details
- Sample Guidelines
- Demo
- FAQ
Customised Exosome Isolation and Proteomics Services
At Creative Proteomics, we offer flexible exosome isolation solutions tailored to the characteristics of your sample type. Depending on sample complexity and project goals, we employ methods such as filtration-centrifugation, commercial exosome isolation kits, or ultracentrifugation to maximise yield and purity.
Once isolated, high-purity exosome particles can be used for downstream proteomics. Our customised exosome proteomics analysis service enables the identification and quantification of exosome-associated proteins, helping researchers uncover biomarkers, signalling pathways, or therapeutic targets.
Service Workflow Overview
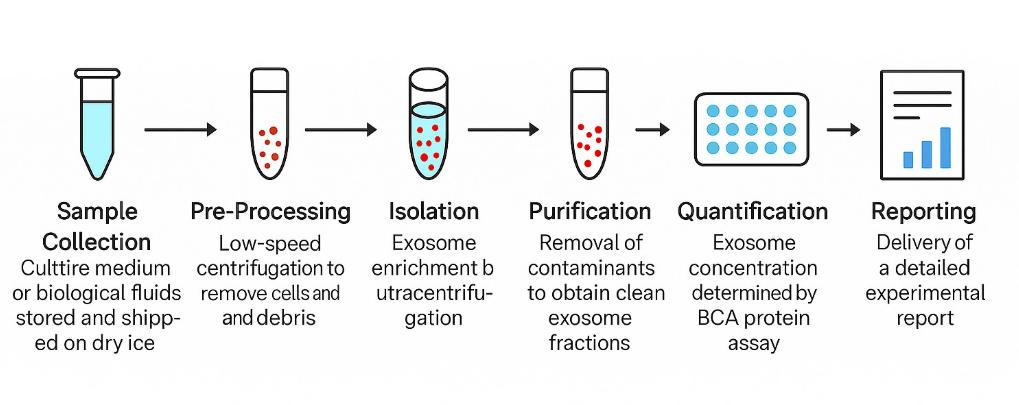
Our exosome isolation and analysis pipeline includes the following steps:
- Sample collection – Culture medium or biological fluids, stored and shipped on dry ice
- Pre-processing – Low-speed centrifugation to remove cells and debris
- Isolation – Exosome enrichment by ultracentrifugation
- Purification – Removal of contaminants to obtain clean exosome fractions
- Quantification – Exosome concentration determined by BCA protein assay
- Reporting – Delivery of a detailed experimental report
Choosing the Right Exosome Isolation Method: Comparative Insights
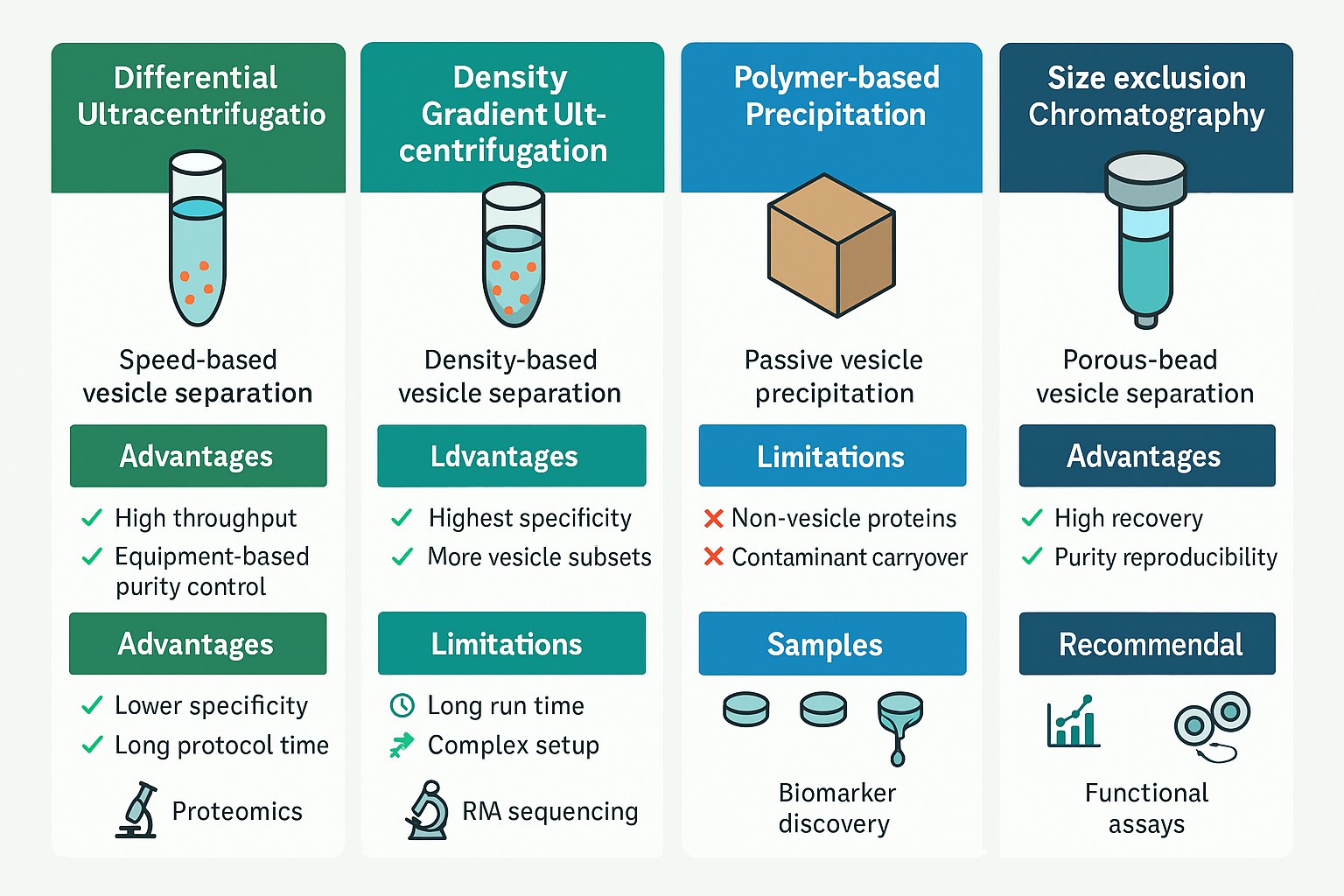
Creative Proteomics offers multiple exosome isolation platforms to accommodate diverse research needs, sample types, and budget considerations. Each method presents distinct strengths and trade-offs. The table below outlines key differences to help you select the most appropriate approach for your project.
| Method | Advantages | Limitations | Recommended For |
|---|---|---|---|
| Differential Ultracentrifugation | Widely established; scalable; compatible with large sample volumes | Time-consuming; lower purity; may co-isolate protein aggregates | High-throughput processing of culture media, urine, or plasma |
| Density Gradient Ultracentrifugation | Highest purity; minimizes protein and vesicle contamination | Complex setup; longer run time; requires gradient materials | Proteomics or RNA sequencing requiring high-purity vesicles |
| Polymer-based Precipitation (e.g., PEG kits) | Fast and easy; no specialised equipment required | Co-precipitation of non-vesicular proteins; not ideal for omics | Early-stage discovery, limited samples, budget-sensitive projects |
| Size Exclusion Chromatography (SEC) | Gentle on vesicle integrity; reproducible and scalable | Requires pre-filtration; column cost may add up | Functional studies; clinical samples where vesicle structure must be preserved |
| Immunoaffinity Capture | High specificity for defined exosome subpopulations (e.g., CD63+, CD81+) | Lower yield; higher cost; limited to known surface markers | Biomarker validation; studies targeting tumour- or tissue-specific exosomes |
Method Selection: Client-Focused Scenarios
- Working with large volumes of conditioned medium?
→ Differential ultracentrifugation offers a balance of cost and scalability. - Planning LC-MS/MS or small RNA sequencing?
→ Density gradient ultracentrifugation ensures the highest analytical purity. - Tight on budget or working with limited volume?
→ Precipitation kits provide a quick and affordable solution, with caveats on purity. - Need intact vesicles for functional assays or EV uptake studies?
→ SEC is ideal for preserving native membrane structure. - Investigating disease-specific exosome markers (e.g., cancer, CNS)?
→ Immunoaffinity capture allows precise enrichment of vesicles expressing target proteins.
Our Added Value
At Creative Proteomics, we don't just isolate exosomes—we guide you in selecting the method that best aligns with your scientific goals. All isolation strategies are fully compatible with our downstream platforms, including:
- NTA (Nanoparticle Tracking Analysis)
- TEM (Transmission Electron Microscopy)
- Western Blot & ELISA
- Exosome proteomics and exosome lipidomics
Whether your priority is purity, scalability, or specificity, we help you make data-driven decisions for optimal results.
High-Purity Exosome Extraction from Diverse Samples
Our team uses ultracentrifugation protocols optimised for a wide range of biological fluids, including:
- Cell culture supernatants
- Serum and plasma
- Urine
- Cerebrospinal fluid
- Additional matrices: ascites, amniotic fluid, breast milk, and saliva
Sample Preparation Guidelines for Exosome Isolation
Accurate and reproducible exosome isolation begins with well-prepared samples. Creative Proteomics provides detailed recommendations for different biological sources to help clients minimize background interference and preserve exosome integrity during collection and storage.
1. Cell Culture Supernatant
To reduce contamination from bovine exosomes, always use media containing exosome-depleted fetal bovine serum (FBS) or serum-free formulations.
Preparation Steps:
- Grow cells to ~70% confluence in standard culture media.
For adherent cells:
- Discard the old medium, rinse with PBS, and replace with exosome-depleted or serum-free medium.
For suspension cells:
- Pellet cells at 300 × g, 4°C for 10 minutes, wash with PBS, and resuspend in fresh exosome-free or serum-free medium.
- Continue culturing for 24–48 hours, adjusting the duration based on cell growth rate.
- Collect supernatant, centrifuge at 300 × g, 4°C for 10 minutes, and carefully remove the supernatant to avoid disturbing cells.
- Filter the supernatant through a 0.22 µm filter to eliminate cell debris, apoptotic bodies, and microvesicles.
- Freeze the cleared supernatant at −80°C.
Recommended volume: ≥20 mL
2. Plasma
Preparation Steps:
- Collect blood in EDTA anticoagulant tubes and gently mix.
- Centrifuge at 2500 × g, 4°C for 15 minutes.
- Carefully transfer the supernatant and repeat centrifugation under the same conditions.
- Aliquot the resulting plasma and freeze at −80°C.
Recommended volume: ≥2 mL
3. Serum
Preparation Steps:
- Collect 4 mL of whole blood in a non-anticoagulant tube.
- Allow to clot at 37°C for 20–30 minutes.
- Centrifuge at 2000 × g for 10 minutes and collect the clear supernatant (serum).
- Store at −80°C.
Recommended volume: ≥2 mL
4. Urine
Preparation Steps:
Collect 50 mL of first-morning urine in a sterile container.
Add a protease inhibitor mix:
- 1.67 mL of 100 mM NaN₃
- 2.5 mL of PMSF (2 mg/mL in isopropanol)
- 50 µL of Leupeptin (1 mg/mL in ddH₂O)
- Process immediately or freeze at −80°C.
Recommended volume: ≥20 mL
5. Cerebrospinal Fluid (CSF)
Preparation Steps:
- Collect CSF and keep on ice for no longer than 30 minutes.
- Centrifuge at 2000 × g for 10 minutes at 4°C to remove cells or debris.
- Transfer the supernatant and store at −80°C.
Recommended volume: ≥10 mL
Applications of Exosome Isolation and Analysis
Exosome profiling has become an essential tool across diverse biomedical and pharmaceutical research domains. Creative Proteomics supports a wide range of application scenarios, including:
- Biomarker Discovery – Identify exosome-associated proteins or miRNAs in cancer, neurodegeneration, or autoimmune diseases
- Liquid Biopsy Development – Profile circulating EVs from serum or plasma for non-invasive diagnostic platforms
- Cell Communication Studies – Investigate the role of exosomes in intercellular signaling under physiological or pathological conditions
- Therapeutic Development – Characterize engineered or drug-loaded exosomes as delivery vehicles
- Response Monitoring – Track exosomal content changes following drug treatment, stress, or environmental exposure
What You Will Receive: Service Deliverables
Every completed project includes a comprehensive data package tailored to your selected analysis type. Standard deliverables include:
- Nanoparticle Tracking Analysis (NTA): Particle size distribution and concentration
- Transmission Electron Microscopy (TEM): Vesicle morphology imaging (if requested)
- Protein Quantification: Total exosomal protein by BCA assay
- Western Blot (optional): Confirmation of exosomal markers (e.g., CD63, CD9, TSG101)
- Proteomics or miRNA Profiling (if selected): Annotated data tables (Excel), figure-ready result summaries (PDF), and analysis notes
DEMO

Frequently Asked Questions (FAQ)
Do I need to use exosome-depleted serum for cell culture experiments?
Yes. To avoid contamination from bovine-derived exosomes, we strongly recommend using exosome-depleted FBS or serum-free media during the final conditioning period.
Can you handle clinical or low-abundance samples like cerebrospinal fluid or amniotic fluid?
Absolutely. We support a wide range of human and animal sample types, including low-volume or rare biological fluids. Please consult us for volume recommendations.
Are your exosome preparations suitable for downstream omics analysis?
Yes. Our workflows are optimised for compatibility with proteomics, small RNA sequencing, and other high-resolution analyses. High-purity options such as density gradient ultracentrifugation and SEC are available.
Is it possible to request specific downstream analysis, like only TEM or only proteomics?
Yes. We offer flexible modular services—clients can select full workflows or choose stand-alone assays like NTA, TEM, Western blotting, or LC-MS.
I'm unsure which isolation method fits my project. Can you help me decide?
Definitely. Our scientific team will evaluate your sample type, goals, and budget to recommend the most appropriate isolation and analysis strategy.
Learn about other Q&A.







