Myocardial infarction (MI) is a type of coronary artery disease that occurs on the basis of atherosclerotic stenosis of the coronary arteries. When a coronary artery ruptures due to certain triggers, platelets in the blood gather on the surface of the ruptured plaque, forming a clot (thrombus) that suddenly blocks the lumen of the coronary artery, leading to ischaemic necrosis of the myocardium. In addition, acute myocardial infarction can be triggered by a dramatic increase in myocardial oxygen consumption or sustained spasm of the coronary arteries.
Proteomics studies can provide data to support the study of the pathogenesis of myocardial infarction and the discovery of therapeutic targets.
Case 1 Exploring mechanisms of scar size regulation after myocardial infarction (1)
Following acute myocardial infarction, the dead myocardium is replaced by scar tissue. Clinical studies have shown that scar size in patients with myocardial infarction is an independent predictor of mortality and prognosis, but little is known about the factors that regulate scar size.
The authors successfully constructed mice with ischaemic myocardial injury and collected cardiac scar tissue and normal heart tissue for RNA sequencing and transcriptomic analysis at 3, 7, 14, 21 and 42 days after ischaemic injury, respectively. In view of the significant high expression of a group of collagen genes at day 7, the authors performed proteomic assays on both the scarred and normal mouse heart tissues collected at day 7. The proteomic results further confirmed the high expression of a group of collagen proteins in the scar tissue. This was subsequently validated by knockdown.
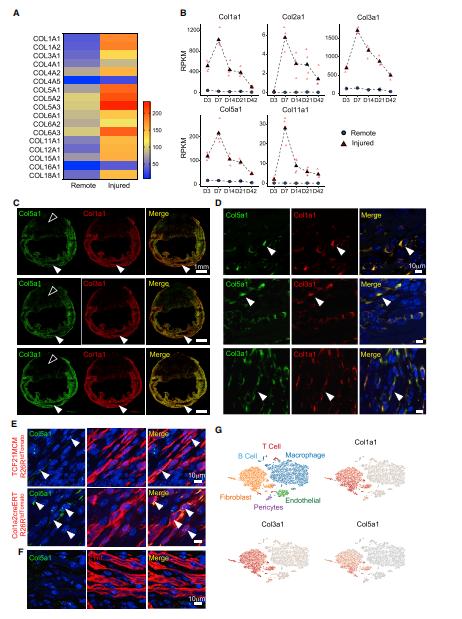
Expression of Col5a1 in Relation to Col1a1 and Col3a1
This study identifies collagen as a key driver of post-traumatic cardiac function. Collagen deficiency alters the mechanical properties of scar tissue, altering matrix and cellular interactions such as inducing mechanosensitive integrin expression, which drives fibroblast activation and increases scar size.
Case 2 An investigation into the mechanisms of scar size regulation after myocardial infarction (2)
Extracellular vesicles (EVs) are submicron, membrane-encapsulated particles that are released from cells in various pathophysiological states. The molecular cargo of these vesicles is thought to reflect the composition of the cell of origin and the EV proteome is therefore a potential source of biomarkers for a variety of diseases. In cardiovascular disease (CVD) studies, plasma EV has been shown to be associated with various cardiovascular risk factors.
Using the CVD II PEA assay panel, 92 low abundance plasma proteins associated with cardiovascular disease were quantified in EV lysates and plasma samples from patients with st-segment elevation myocardial infarction (STEMI, n = 60) and healthy controls (CTRL, n = 22). Fifty-two proteins were detected in EV lysates, i.e. present in 50% of the samples from the STEMI or CTRL groups. All 92 proteins were detected in plasma samples. Hierarchical clustering and principal component analysis based on the levels of all detected proteins showed that, with few exceptions, STEMI patients and CTRL individuals were clustered separately. Based on these results, the authors found that a general perturbation of the EV and plasma proteomes occurred during myocardial infarction.
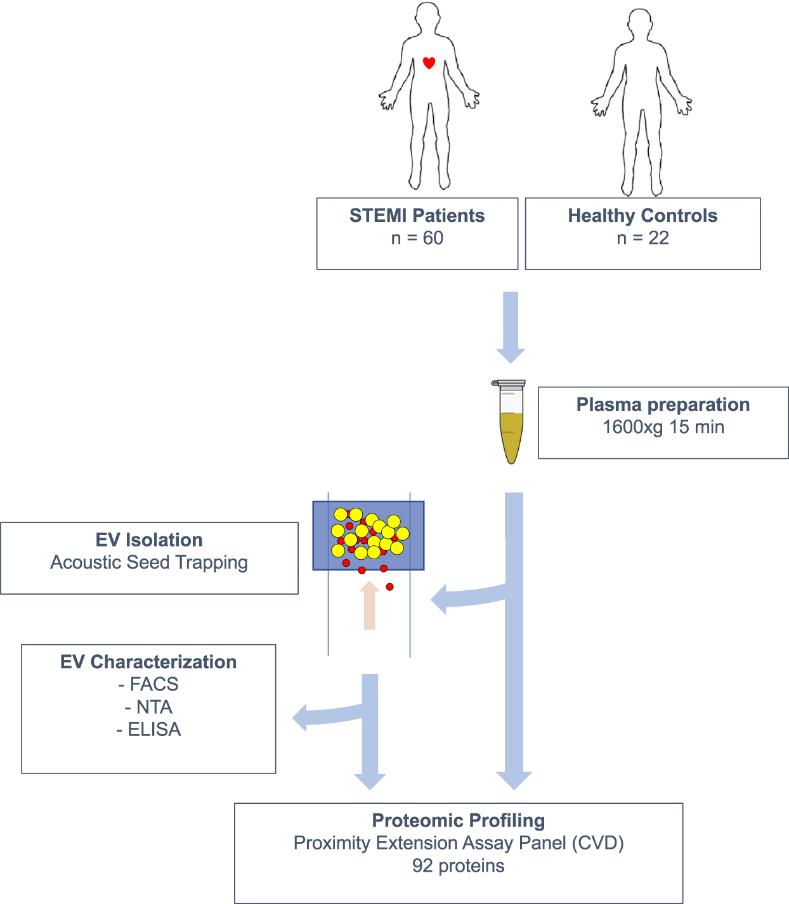
Overview of the study design.
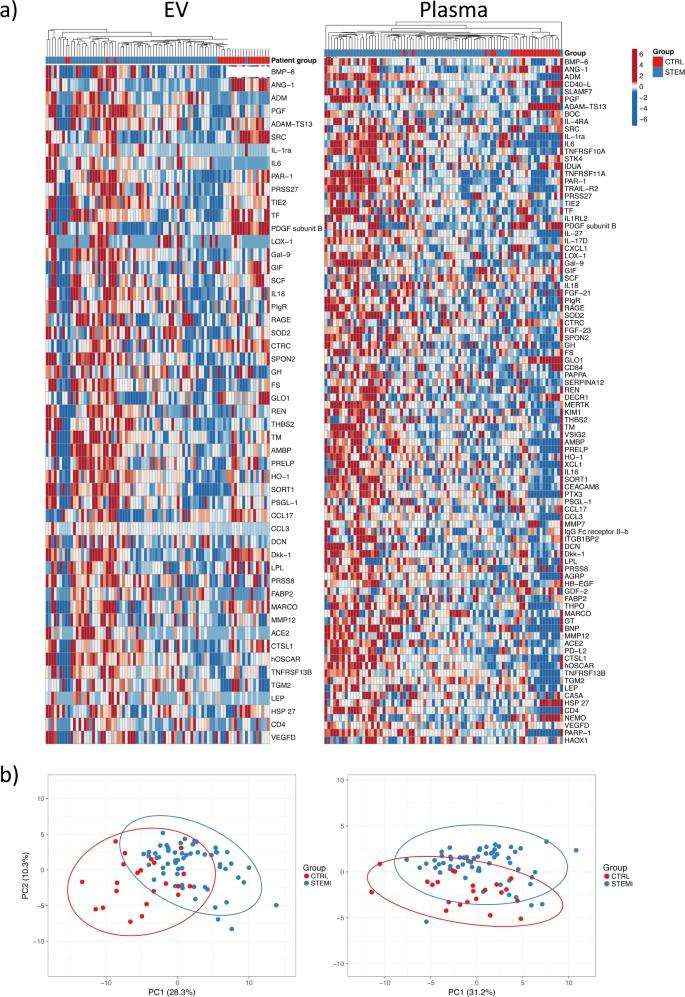
Proteomic profiling of EV lysates and plasma.
(a) Heatmaps of all detected proteins in EV lysates (n = 52) and plasma (n = 92) across all samples. (b) Principal component analysis based on all proteins detected in EV lysates and plasma.
To assess whether EV protein levels differed from those in plasma, the authors also analysed and compared the relative levels of 52 EV proteins in the sample types and found similarities in the low abundance of proteins detected in the two fractions. The proteins differentially expressed in patients and controls were subsequently identified and validated.
The 3 proteins that were dysregulated in the EV fraction but not in plasma were chemokine (C-C motif) ligand 17 (CCL17), chymotrypsin C (CTRC) and proto-oncogene tyrosine-protein kinase SRC (SRC), and all three were reduced in patients (between 1.2- and 2-fold). EV-associated SRC, but not plasma SRC, is a marker of advanced coronary artery disease, but is not specific for MI.
Reference
- Yokota, Tomohiro, et al. "Type V collagen in scar tissue regulates the size of scar after heart injury." Cell 182.3 (2020): 545-562.
- Gidlöf, Olof, et al. "Proteomic profiling of extracellular vesicles reveals additional diagnostic biomarkers for myocardial infarction compared to plasma alone." Scientific Reports 9.1 (2019): 8991.






