1 SILAC Labeling
a) Propagate the control and stimulated cell line(s) to be compared in a particular medium (e.g., DMEM). One cell line should be grown in medium containing light amino acids, whereas the other(s) should be grown in media containing one of the heavy amino acid combinations listed in Table 1.
Table 1 Recommended heavy amino acids for multiplexed SILAC experiments
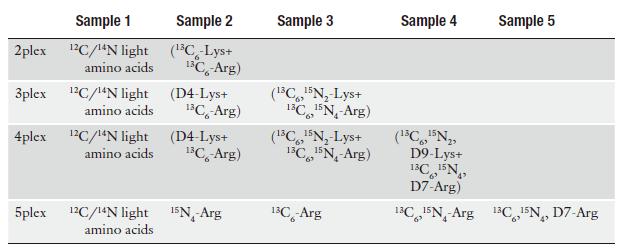
b) Each cell line must first be adapted to ensure complete incorporation prior to initiating the experiment. In general, five passages are sufficient for this purpose.
2 Cell Lysis
a) Replace the medium with serum free medium and leave the cells in culture for an additional 12–18 h.
b) Wash the cells twice with PBS and stimulate one of the cell populations with the required amount of the ligand (e.g., EGF).
c) Wash the cells three times with cold PBS.
d) In the case of 150-mm cell culture plates, place these on ice and rinse them with 15 mL of pre-chilled lysis buffer, which should be freshly prepared and with phosphatase inhibitors.
e) Dislodge the cells gently from the culture plate with a disposable cell scraper and place the liquid in a 50-mL centrifuge tube. Allow the tube to rock slowly for an hour at 4°C.
f) Centrifuge the cell lysate at 12,000 × g at 4°C to remove the insoluble debris.
3 Acetone Precipitation
a) Precipitate the proteins from the lysate with a 5× volume of cold acetone, at −20°C, overnight.
b) Recover the precipitate by spinning the acetone/lysate mixture at (12,000–15,000 × g) for 15 min at 4°C.
c) Remove the supernatant carefully and leave the pellet to dry in a clean hood.
d) Recover the protein pellet with 200 μL of dissolution buffer and incubate the sample at 30°C with constant mixing and occasional vortexing.
e) Measure the total protein concentration for each sample with the Bio-Rad Protein assay following the manufacturer's instructions.
f) Prepare aliquots of 100–200 μg total protein in 1.5-mL micro centrifuge tubes.
g) Flash-freeze the samples in liquid nitrogen, and store them at −80°C if they are not to be used immediately.
h) Reduce, alkylate and use for in-solution trypsin digestion (5).
4 SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
a) For each sample, mix 100–200 μg of total protein in a volume of 10–15 with 5 μL of SDS-PAGE loading buffer.
b) Heat the samples at 95°C for 5 min.
c) Allow the samples to cool at room temperature for 5 min.
d) Load the samples onto a pre-cast mini-gel, assembled on a Novex mini-cell with SDS-PAGE running buffer.
e) Perform electrophoresis at 200 V until the loading buffer stain reaches the bottom of the gel.
f) Transfer the gel to a clean and covered containe.
g) Wash the gel with H2O twice briefly.
h) Stain the gel with colloidal Coomassie blue.
5 Reduction and Alkylation
a) Excise each gel lane in small pieces of about 1 mm.
b) Transfer the gel cubes in a clean 1.5-mL micro centrifuge tube.
c) Destain the gel pieces with 1:1 of a 50 mM NH4CO3/acetonitrile mixture for at least 2 hours.
d) Wash the samples step-wise with H2O and H2O/acetonitrile for 15 min each time. Repeat this step once.
e) Dehydrate the gel pieces with acetonitrile 100% for 15 min.
f) Swell the dehydrated gel pieces with 1 mL of reduction solution, and incubate for 60 min at 60°C.
g) Leave the reaction at room temperature to cool down.
h) Discard the reduction solution.
i) Add 1 mL of alkylation solution per tube, incubating the samples at room temperature and protected from light, for 30 min.
6 In-Gel Protein Digestion
a) Wash the samples step-wise with H2O and H2O/acetonitrile.
b) Dehydrate the gel pieces with acetonitrile 100%.
c) Dissolve a lyophilized vial of trypsin with pre-chilled 50 Mm NH4CO3 at a concentration of 10–15 ng/μL.
d) Swell the dehydrated gel pieces with freshly dissolved trypsin on ice, using a volume slightly beyond that required to cover the dry gel pieces in each tube.
e) Incubate the samples for 45 min on ice.
f) Remove the excess of liquid, and replace it with approximately the same volume of 50 mM NH4CO3, but without trypsin.
g) Allow the reaction to proceed at 37°C for 12–14 h.
h) Stop the trypsin digestion tube with a 1:5 v/v aliquot of 10% acetic acid.
i) Transfer the supernatant to a clean 1.5-mL micro centrifuge tube.
j) Extract the peptides remaining in the gel matrix three times by incubating with 0.1% acetic acid, 0.1% acetic acid in 50% acetonitrile and 100% acetonitrile, respectively.
k) For each sample, transfer the peptides from above wash and dehydration steps to the tube in which the supernatant was collected Subheading 6, Step h.
l) Dry each sample using vacuum centrifugation.
7 Affinity Enrichment Using TiO2
a) Redissolve the dried samples in 1 mL of TiO2 loading solution.
b) Apply vigorous vortex to assure the recovery of the sample and spin briefly to remove insoluble particles.
c) Transfer 10 μL of a 1/1 H2O/TiO2 slurry per sample to a 1.5-mL clean micro centrifuge tube.
d) Make a suspension with the TiO2 slurry in each tube, by adding 1 mL of TiO2 loading solution.
e) Equilibrate the TiO2 slurry for 15 min with slow rocking.
f) Spin the TiO2 slurry briefly at low speed.
g) Discard the TiO2 loading solution used for equilibration.
h) To each of the redissolved samples as in Subheading 7, step a, add 10 μL of equilibrated TiO2 slurry.
i) Allow the phosphopeptides to bind to the TiO2 material for 30 min in slow rocking.
j) Spin the samples briefly.
k) Transfer the supernatant, which is the nonbound peptide solution to a 1.5-mL clean micro centrifuge tube.
l) Carefully redissolve the TiO2 pellet in 100 μL of TiO2 washing solution.
m) Incubate the above slurry for 15 min with slow rocking.
n) Repeat the previous step three times.
o) Carefully dissolve the TiO2 pellet with 100 μL of 25% NH4OH at pH 10.5.
p) Incubate the slurry for 15 min with slow rocking.
q) Repeat the previous step once more to assure complete elution of the bound peptides.
r) Bring the pH of the 200 μL of phosphopeptide elution to pH 7.5 by adding appropriate amount of 10% acetic acid.
s) Dry the samples obtained in Subheading 7, step k (nonbound peptide solution) and Subheading 3.7, step r (phosphopeptide elution) in a vacuum centrifuge.
t) Dissolve peptides in LC-MS solvent and use for phosphopeptide analysis.
8 Strong Cation Exchange (SCX) Chromatography
a) The in-solution digested samples enriched with TiO2 are redissolved in 1 mL of SCX-loading solution.
b) Inject the sample onto the SCX column and start the chromatographic experiment.
c) For an off-line LC-system coupled to an auto-sampler, the SCX gradient is as follows:
– 70 min run starting with 0% solution B
– 0–20 min, isocratic 0% solution B
– 20–22 min, 0–8% solution B
– 22–48 min, 8–35% solution B
– 48–58 min, 35–100% solution B
– 58–68 min, isocratic 100% solution B
– 68–69.5 min, 100–0% solution B
– 69.5–70 min, 0% solution B End
Peptides can be fractionated using strong cation exchange chromatography on PolySULFOETHYL A column (PolyLC, Columbia, MD) (100 × 2.1 mm, 5-μm particles with 300 Å pores). SCX chromatography can be performed using Ultimate HPLC system (LC Packings) connected to a Probot fraction collector. SCX fractions (0.5 mL) can be collected using a nonlinear gradient of 0–350 of SCX solution B. First 20 min, SCX solution A is injected for cleaning SCX bound peptides and subsequently fractionation is done using 0–35% SCX solution B for 30 min, increasing solution B to 100% for further 15 min duration.).
d) Collect the elution aliquots in several fractions, depending on the chromatographic profile.
e) Dry each SCX fraction using vacuum centrifugation.
9 Peptide Desalting
The final step before LC-MS/MS is to desalt the SCX fractions by batchwise reversed-phase chromatography with Poros 20 R2 material.
a) Redissolve the dried samples obtained above in Subheading 8, step e with 1 mL of loading RP-C solution.
b) Apply vigorous vortex to assure the recovery of the sample.
c) Transfer a small amount of Poros 20 R2 per sample to a 1.5 mL clean micro centrifuge tube.
d) Make a suspension with the Poros 20 R2 in each tube, by adding 1 mL of RP-C loading solution.
e) Equilibrate the Poros 20 R2 material for 15 min with slow rocking.
f) Spin the Poros 20 R2 slurry briefly at low speed.
g) Discard the loading solution used for equilibration.
h) Add each of the redissolved samples to an aliquot of equilibrated Poros 20 R2.
i) Incubate for 1 h and slow rocking.
j) Spin the samples briefly.
k) Discard the supernatant.
l) Carefully redissolve the Poros 20 R2 pellets in 100 μL of RP-C washing solution.
m) Incubate for 15 min with slow rocking.
n) Repeat the previous step three times.
o) Carefully dissolve the Poros 20 R2 pellet with 100 μL of RP-C elution solution.
p) Incubate the slurry for 15 min with slow rocking.
q) Repeat the previous step once more to assure complete elution of the bound peptides.
r) Dry each desalted sample with a vacuum centrifuge.
s) When needed, redissolve the samples in an appropriate LC-MS/MS loading solution.
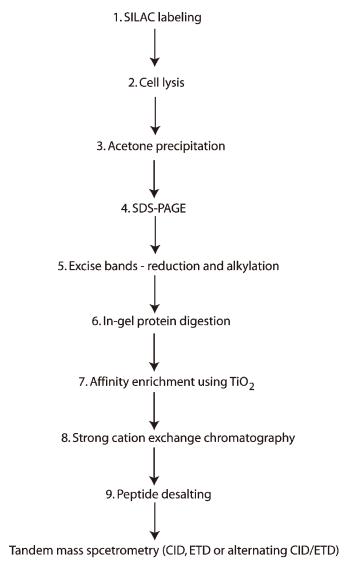
A schematic of the steps involved in a representative SILAC-based phosphoproteomic analysis using TiO2.
Reference
- de Graauw, M. (2009). Phospho-Proteomics. Humana Press.






