Melanoma is highly dangerous and patients' conditions can rapidly worsen. There are few effective medications available and treatment outcomes are poor. According to statistics, the 5-year survival rate for melanoma patients with lymphatic metastasis is only 24% to 70%, and for those with distant metastasis, the 5-year survival rate is less than 10% and the melanoma is often incurable. Although melanoma is usually easier to detect earlier than other types of cancer, it is highly likely to spread or metastasize to other tissues/organs in the body.
Proteomics is a critical tool in the study of melanoma, a type of skin cancer that is known for its aggressive behavior and poor prognosis. Through the use of high-throughput technologies, such as 2D gel electrophoresis and mass spectrometry, researchers can analyze the protein expression profile of melanoma cells and tissues. This information is crucial for uncovering new insights into the biology of melanoma, including the identification of novel therapeutic targets and biomarkers. For example, proteomic analysis of melanoma has led to the discovery of new drug targets, such as the gene encoding the protein B-Raf, which is frequently mutated in melanoma. Additionally, proteomic analysis of melanoma tissues has revealed new biomarkers for early detection and disease progression, as well as potential predictors of response to treatment. These findings have significant implications for the development of more effective and personalized therapies for melanoma patients.
Case 1 Proteomic screening for signature proteins in early AD (1)
Immunotherapy has brought about a major change in the treatment strategy for metastatic melanoma patients, with seven FDA-approved immunotherapies currently being used in the clinical treatment of melanoma. Melanoma is more likely to improve patients' survival rates compared to other cancers, partly due to its higher mutational load. Immunotherapy with checkpoint inhibitors (such as PD-1) and TIL (tumor-infiltrating lymphocyte) immunotherapy has shown good therapeutic effects, but still, nearly 50% of patients are non-responsive or insensitive to immunotherapy.
The authors conducted a detailed and comprehensive analysis at the proteomic level, selecting a patient population of 42 receiving TIL therapy and 74 receiving PD-1 therapy. Deep proteomic analysis was carried out using FFPE samples from the above patients.
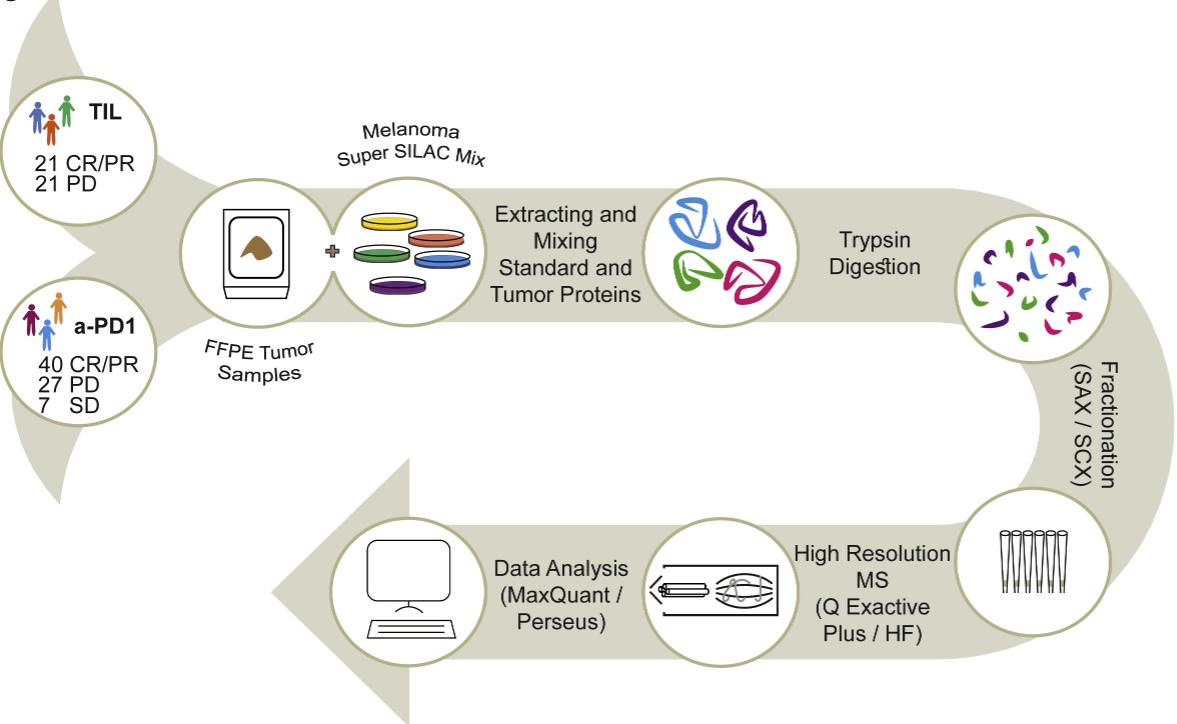
The proteomics workflow involved protein extraction from FFPE tissues and mixing with a super-SILAC standard.
KEGG pathway analysis of differential proteins and two-dimensional annotation enrichment analysis revealed that the enrichment of the two immunotherapies was relatively minuscule. Immunotherapy responders were dominated by metabolism-related proteins, while non-responders were dominated by spliceosomal, RNA-metabolism-related proteins. In addition, both datasets had a higher proportion of antigen presentation-related proteins in responders, as well as proteins related to signalling pathways that play an important role in the immunotherapeutic response to melanoma (e.g. MAPK, NF-kB and RAS signalling pathways).
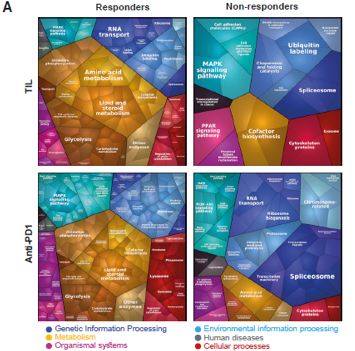
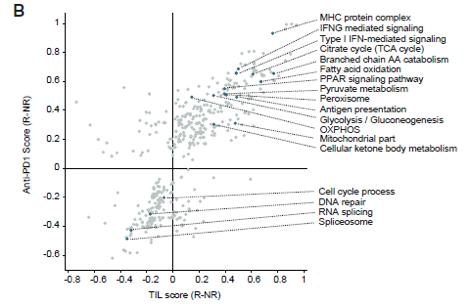
Functional annotation of differential proteins in anti-PD-1 and TIL therapy responders and non responders
Subsequently, the correspondence between functional determinants and clinical parameters was analysed using WGCNA (weighted gene correlation network analysis). Integrative analysis of data from the population receiving both therapies (no therapy, only response and non-response) revealed that differential proteins were associated with antigen presentation, IFN and regulation of mitochondrial metabolism. Patients responding and non-responding to the different immunotherapies had their own indication proteins (8 for TIL and 5 for PD-1) and these were strongly associated with progression-free survival.
Case 2 Large-scale deep multi-layer analysis of Alzheimer's disease brain (2)
Using the plasma proteomics tool, Massachusetts General Hospital investigators performed plasma proteomic analysis (>700 proteins) in 174 patients with metastatic melanoma treated with ICB at three time points. The study utilized an independent discovery and validation cohort to establish a combination of immunotherapy response predictors superior to those based on tissue methodology.
Two hundred seventeen differentially expressed proteins were identified between ICB responders (Responders, R) and non-responders (Non-responders, NR), including a co-regulatory protein module that was enriched in some NR patients. The relative contribution of tumor cells to circulating proteins was resolved by analyzing single-cell RNA sequencing data from tumor biopsies of 32 patients. The majority of proteins in the co-regulatory NR module were derived from tumor cells and myeloid cells. In myeloid cells, the investigators identified a group of Tumor-Associated Macrophages (TAMs) with a suppressor phenotype that express the co-regulatory NR module at high levels, thus suggesting that they are key drivers of non-response. In conclusion, this data demonstrates the utility of plasma proteomics in biomarker discovery and in understanding the biology of the organism's response to tumors, and can be applied to guide patient stratification in the clinical treatment of melanoma in the ICB for early identification of drug-appropriate groups/individuals for precise dosing.
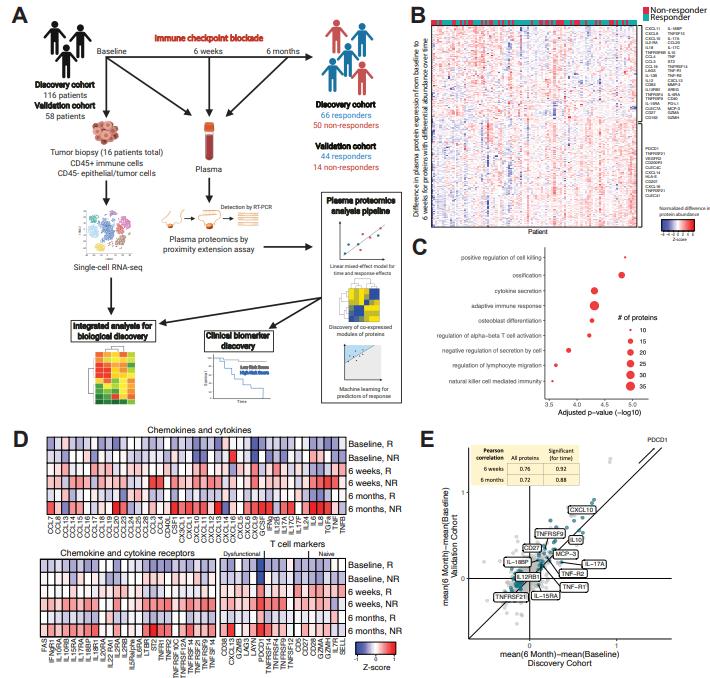
Melanoma patients treated with ICB have a unique treatment-associated pattern of inflammatory plasma proteins over time.
Reference
- Harel, Michal, et al. "Proteomics of melanoma response to immunotherapy reveals mitochondrial dependence." Cell 179.1 (2019): 236-250.
- Mehta, Arnav, et al. "Plasma proteomic biomarkers identify non-responders and reveal biological insights about the tumor microenvironment in melanoma patients after PD1 blockade." bioRxiv (2022): 2022-02.





