S-Nitrosation, a dynamic nitric oxide (NO)-mediated post-translational modification (PTM), modulates protein functionality, signaling cascades, and oxidative stress responses through the covalent attachment of NO to cysteine sulfhydryl (-SH) groups, forming S-nitrosothiol (-SNO) adducts. However, the transient nature, low stoichiometry, and chemical lability of this modification present significant challenges for its precise identification and quantification.
This review provides a comprehensive overview of contemporary detection strategies, focusing on three pivotal approaches: (1) advanced mass spectrometry (MS) workflows for site-specific resolution, (2) chemical derivatization techniques to stabilize and enrich -SNO moieties, and (3) bioinformatics tools for predictive modeling and data interpretation. Practical recommendations for experimental design, including sample preparation protocols and analytical workflows, are integrated to guide researchers in overcoming technical limitations and enhancing reproducibility.
By bridging methodological innovations with biological applications, this work aims to advance the study of S-nitrosation's role in health and disease.
 Common methods for SNO detection (Wang H et al., 2011)
Common methods for SNO detection (Wang H et al., 2011)
Analytical Challenges and Strategic Advances in S-Nitrosation Detection
1. Chemical Lability of S-Nitrosothiols
- Primary Obstacle: S-Nitrosothiol (SNO) modifications exhibit rapid degradation under exposure to light, elevated temperatures, redox-active metal ions (e.g., Cu²⁺, Fe³⁺), and reducing agents (e.g., ascorbate).
Strategic Solutions
- Optimized Handling Protocols:
- Conduct experiments in light-restricted,Low temperature condition (4°C or snap-freezing in liquid N₂).
- Incorporate metal chelators (e.g., EDTA) to inhibit metal-catalyzed degradation.
- Pre-treat samples with alkylating agents (e.g., N-ethylmaleimide) to block free thiols, preventing SNO reduction or artifactual nitrosation.
- Stabilization Approaches:
- Employ mercury-based stabilizers (e.g., HgCl₂) or chemical "locking" via succinylation (e.g., SNOAC method).
- Implement in situ detection tools (e.g., fluorescent probes like DAN or genetically encoded SNO biosensors) to minimize processing delays.
2. Low Physiological Abundance of SNO Modifications
- Primary Obstacle: SNO-modified proteins typically constitute<1% of total cellular proteins, evading conventional detection limits.
Strategic Solutions
- Enrichment Strategies:
- Biotin Switch Technique (BST): Convert SNO groups to biotin tags for streptavidin-based enrichment.
- Click Chemistry: Leverage bioorthogonal reactions (e.g., azide-alkyne cycloaddition) for selective SNO labeling.
- High-Sensitivity Methodologies:
- Amplify signals using nanoparticle-enhanced immunoassays (e.g., AuNPs, quantum dots).
- Deploy ultra-sensitive platforms like droplet digital PCR or single-molecule arrays (Simoa).
- Scale up initial protein inputs (mg-range) combined with prefractionation (e.g., SDS-PAGE).
3. Site-Specific Identification of SNO Modifications
- Primary Obstacle: Discriminating adjacent cysteine residues (e.g., Cys123 vs. Cys125) within the same protein.
Strategic Solutions
- Advanced Mass Spectrometry:
- Utilize electron-transfer dissociation (ETD) to preserve labile PTMs during peptide fragmentation.
- Integrate ion mobility spectrometry (IMS) for enhanced separation of isobaric peptides.
- Targeted Validation:
- Engineer site-directed mutants (Cys→Ser/Ala) to confirm modification loci via functional assays.
- Develop epitope-specific antibodies (caveat: cross-reactivity risks with neighboring residues).
- Computational Prediction: Apply algorithms (e.g., GPS-SNO) to prioritize candidate sites for experimental validation.
Integrated Approaches for Comprehensive Analysis
- Dynamic Profiling: Use photoactivatable NO donors (e.g., caged NO compounds) for real-time tracking of SNO flux.
- Multimodal Verification: Correlate mass spectrometry data with immunoblotting and functional readouts (e.g., enzymatic activity assays) to ensure robustness.
- Clinical Translation: Optimize protocols for complex biofluids (e.g., plasma, CSF) by addressing matrix interference and SNO transient stability.
Mainstream detection method and technical process
Biotin Switch Technique (BST)
1. Technical Principles and Protocol Refinement
- Core Concept: The BST converts labile S-nitrosothiol (-SNO) modifications into stable biotinylated adducts through sequential chemical reactions, enabling enrichment and detection.
Stepwise Protocol Optimization
- Alkylation of Unmodified Thiols
- Reagent Selection:
- MMTS: Forms reversible mixed disulfides (-S-S-Me) but may incompletely block accessible thiols.
- NEM: Irreversibly alkylates free thiols, offering superior blocking efficiency but potential interference with downstream steps.
- Reaction Parameters:
- Concentration: 1 mM MMTS balances efficacy with protein solubility.
- Duration: 30 minutes at 25°C (extend to 60 minutes for complex matrices, monitoring protein integrity).
- Reagent Selection:
- Selective Reduction of SNO Groups
- Reduction Strategies:
- Ascorbate: Reduces -SNO to -SH at 1–5 mM; higher concentrations risk nonspecific disulfide bond cleavage.
- Cu⁺-Assisted Reduction: Enhances specificity but requires light-protected conditions.
- Reduction Strategies:
- Critical Controls:
- Dark-phase operations to prevent ascorbate-derived ROS generation.
- Low-temperature incubation (4°C) to minimize background reactivity.
- Biotinylation and Enrichment
- Labeling Reagents:
- HPDP-Biotin: Maleimide-PEG2-biotin conjugates with minimal steric hindrance for nascent thiols.
- Alternative Tags: IAA-biotin requires alkaline pH (>8.0) for optimal efficiency.
- Labeling Reagents:
- Conditions:
- Concentration: 0.5–1 mM HPDP-biotin to balance specificity and nonspecific binding.
- Duration: 2-hour incubation prevents excessive background signals.
- Affinity Purification and Detection
- Streptavidin Bead Workflow:
- Pre-Wash: Buffer with 0.1% SDS reduces nonspecific adsorption.
- Elution: 50 mM DTT or β-mercaptoethanol (95°C, 10 minutes) releases biotinylated proteins.
- Streptavidin Bead Workflow:
- Analytical Methods:
- Immunoblotting: High-sensitivity HRP-streptavidin conjugates for direct visualization.
- Mass Spectrometry: LC-MS/MS-compatible workflows with CHAPS/Triton X-100 lysates to avoid SDS interference.
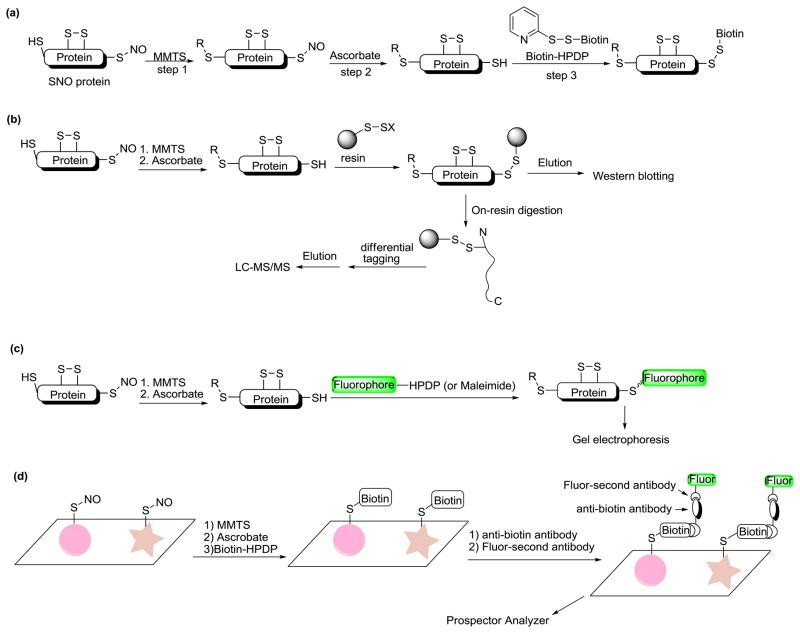 Biotin switch based methods for SNO detection (Wang H et al., 2011)
Biotin switch based methods for SNO detection (Wang H et al., 2011)
2. Advantages, Limitations, and Mitigation Strategies
- Strengths:
- Cost-Effective: Utilizes affordable reagents (MMTS, ascorbate) without specialized instrumentation.
- Versatility: Adaptable to diverse samples (cell lysates, biofluids, tissues).
- Integration: Compatible with proteomic (2D-PAGE) and functional assays (enzyme activity profiling).
- Challenges and Solutions:
- False Positives:
- Causes: Ascorbate-mediated disulfide reduction or incomplete thiol blocking.
- Mitigation: Dual alkylation (MMTS + iodoacetamide) and mutant controls (Cys→Ser).
- Throughput Constraints:
- Automation: 96-well magnetic bead platforms and robotic liquid handlers (e.g., Beckman Biomek).
- Multiplexing: TMT-labeled high-throughput MS workflows.
- Sensitivity Limits:
- Signal Amplification: Tyramine-based enhancement (TSA) or nanoparticle-conjugated streptavidin.
- False Positives:
3. Experimental Best Practices
- Sample Handling:
- Immediate processing or snap-freezing in liquid nitrogen to preserve SNO integrity.
- Avoid repeated freeze-thaw cycles.
- Light Sensitivity Management:
- Conduct all steps under red light or in darkrooms to protect light-labile reagents (ascorbate, HPDP-biotin).
- Control Design:
- Positive Control: GSNO-treated samples to validate SNO detection.
- Negative Controls: Ascorbate omission or DTT pretreatment to confirm specificity.
- MS Compatibility:
- Substitute SDS with MS-friendly detergents (CHAPS, Triton X-100) during lysis.
Services You May Be Interested In:
Antibody-Based Detection
1. Technical Principles and Protocol Design
- Core Concept: Antibodies targeting S-nitrosothiol (-SNO) modifications or site-specific epitopes enable direct detection and enrichment via immunological methods (e.g., immunoblotting, immunoprecipitation, fluorescence imaging).
Key Protocol Components
- Sample Stabilization
- Maintain SNO integrity by incorporating metal chelators (e.g., EDTA) and protease inhibitors to block degradation.
- Snap-freeze samples in liquid nitrogen; avoid repeated freeze-thaw cycles.
- Use non-reducing lysis buffers (exclude β-mercaptoethanol/DTT) to preserve labile -SNO groups.
- Antibody Selection
- Pan-SNO Antibodies:
- Example: Anti-SNO-Cys antibodies (e.g., Sigma-Aldrich) recognize a conserved SNO-cysteine motif.
- Limitation: Potential cross-reactivity with other cysteine oxidations (e.g., S-sulfonation).
- Site-Specific Antibodies:
- Application: Custom antibodies for known SNO sites (e.g., Cys94 in a target protein).
- Drawback: High development costs and restricted applicability.
- Pan-SNO Antibodies:
- Immunoassay Workflows
- Immunoblotting:
- Electrophoresis: Non-reducing SDS-PAGE preserves SNO modifications.
- Blocking: 5% BSA minimizes background vs. skim milk.
- Antibody Titration: Optimize primary antibody dilution (1:500–1:2000) to reduce nonspecific binding.
- Immunoprecipitation:
- Enrich SNO-proteins using pan-SNO antibodies, followed by MS-based identification.
- Elution: Mild acidic buffers (pH 2.8) retain modification stability.
- Immunofluorescence:
- Fixation: Methanol/acetone preserves SNO; avoid aldehyde-based fixatives.
- Validation: Co-treatment with NO donors/inhibitors confirms signal specificity.
- Immunoblotting:
2. Strengths and Limitations
- Advantages:
- Exceptional Sensitivity: Detects SNO-modified proteins at picomolar (pM) concentrations.
- Native-State Analysis: No chemical derivatization required, preserving physiological modification states.
- Spatiotemporal Resolution: Enables in situ localization in tissues or cells (e.g., confocal imaging).
- Challenges and Mitigation Strategies:
- Antibody Cross-Reactivity: Epitope overlap with other cysteine oxidations (e.g., disulfides).
- Solutions:
- Validate findings via LC-MS/MS post-immunoprecipitation.
- Perform competitive assays with excess SNO-peptides to block specific signals.
- Include reduction controls (ascorbate/DTT pretreatment) to confirm SNO dependence.
- Limited Antibody Breadth: Most antibodies target specific proteins/sites.
- Workarounds:
- Combine multiple pan-SNO antibodies to broaden coverage.
- Stabilize SNO groups via photo-crosslinkers (e.g., diazirine) to enhance recognition.
- Dynamic Range Constraints: Dominant proteins (e.g., serum albumin) mask low-abundance targets.
- Mitigation:
- Pre-fractionate samples using ion-exchange chromatography or SDS-PAGE.
- Amplify signals with tyramide-based systems (TSA) or nanoparticle conjugates.
3. Critical Experimental Considerations
- Sample Handling Precautions: Avoid mercury-based reagents (e.g., HgCl₂) and heat denaturation, which degrade SNO groups.
- Antibody Management:
- Aliquot and store at -80°C to prevent freeze-thaw degradation.
- Pre-adsorb antibodies with control lysates to minimize nonspecific binding.
- Control Design:
- Positive Control: Spiked SNO-modified standards (e.g., SNO-BSA).
- Negative Controls:
- Ascorbate/DTT pretreatment to abolish SNO signals.
- Isotype-matched IgG to exclude nonspecific interactions.
Mass Spectrometry
1. Advanced Integration of Sample Preparation and Enrichment Strategies
- Objective: Enhance recovery of low-abundance S-nitrosothiol (-SNO) modifications while minimizing background noise.
- Click Chemistry Workflows:
- SNO Stabilization: Add DTPA (1 mM) and operate under light-restricted conditions to inhibit metal-catalyzed degradation.
- Azide Derivatization: Convert -SNO to azide (-S-N₃, +43.01 Da) via Cu(I)-BTTAA catalysis (30-minute reaction to limit side reactions).
- Biotinylation: Utilize strain-promoted azide-alkyne cycloaddition (SPAAC) with DBCO-biotin for copper-free conjugation, achieving enrichment thresholds as low as 0.01%.
- Alternative Enrichment Approaches:
- SNO-RAC: Thiol-reactive resins directly capture reduced -SH groups, bypassing biotinylation steps.
- Immunoprecipitation (IP): Employ high-specificity antibodies (e.g., Anti-SNO-Cys) with rigorous cross-reactivity validation.
- Complexity Reduction via Pre-Fractionation:
- Strong Cation Exchange (SCX): Segregate peptides by charge to reduce co-elution.
- High-pH Reversed-Phase Chromatography (HpH-RP): Fractionate into 10–15 pools to enhance proteomic depth.
2. Optimized Protein Digestion and Peptide Preparation
- Goal: Generate MS-compatible peptides while preserving -SNO stability.
- Enzymatic Digestion:
- Trypsin: Overnight digestion at 37°C yields arginine/lysine-terminated peptides (8–20 aa) ideal for LC-MS/MS.
- Glu-C: 6-hour digestion at 25°C produces glutamic/aspartic acid-terminated peptides for long-modification mapping.
- Additives and Controls:
- Rapigest (0.1%): Enhances solubility of hydrophobic peptides.
- Reduction Alternatives: Replace DTT with TCEP to preserve -SNO groups while reducing non-target disulfides.
3. Mass Spectrometry Instrumentation and Parameter Optimization
- Platform-Specific Tuning:
- Orbitrap Exploris 480:
- Resolution: 240,000 (@ m/z 200).
- Fragmentation: HCD (25–35% energy) with SPS-MS3 for precise quantification.
- TimsTOF Pro 2:
- 4D-Proteomics: parallel accumulation–serial fragmentation acquisition mode with ion mobility (CCS values) to resolve isobaric peptides.
- Quantification: Compatible with 16-plex TMT labeling.
- Q Exactive HF-X:
- Scan Rate: >15 Hz with 30-second dynamic exclusion.
- Orbitrap Exploris 480:
- Acquisition Refinements:
- Hybrid Fragmentation: Combine HCD (b/y ions) and ETD (modification preservation) for confident site assignment.
- Narrow Isolation Windows: 1.2 Da isolation width to reduce co-elution interference.
4. Data Analysis and Validation Frameworks
- Database Searches:
- MaxQuant:
- Dynamic Modifications: +78.98 Da (SNO), +43.01 Da (azide), +15.99 Da (oxidation).
- Fixed Modifications: Cys alkylation (e.g., MMTS, +57.02 Da).
- FDR: ≤1% at PSM and protein levels.
- PEAKS X+: De novo sequencing for low-abundance modifications.
- MaxQuant:
- Quantitative Strategies:
- TMT/iTRAQ: 16-plex labels for comparative SNO profiling (e.g., oxidative stress vs. control).
- SILAC: Metabolic labeling for time-resolved SNO dynamics.
- Label-Free: MaxLFQ for clinical samples (plasma, CSF).
- Validation Approaches:
- Synthetic Peptides: Confirm retention times and fragmentation patterns of SNO-containing sequences.
- Mutants generated by gene editing: Validate sites via Cys→Ser/Ala substitutions in cell models.
- Antibody-MS Cross-Validation: IP enrichment followed by MS verification.
5. Technical Challenges and Integrated Solutions
- Challenge 1: Sensitivity Limits for Rare Modifications
- Solution: Nano-LC systems (75 μm columns, 300 nL/min flow) enhance ionization; TimsTOF ion mobility resolves isobaric ions.
- Challenge 2: False Positives/Negatives
- Solution: Dual database searches (target/decoy) with pDeep3 machine learning to filter implausible spectra.
- Challenge 3: Dynamic Range Constraints
- Solution: Antibody pre-enrichment (e.g., eNOS IP) and DDA/DIA hybrid acquisition for comprehensive coverage.
References
- Ye H, Wu J, Liang Z, Zhang Y, Huang Z. Protein S-Nitrosation: Biochemistry, Identification, Molecular Mechanisms, and Therapeutic Applications. J Med Chem. 2022 Apr 28;65(8):5902-5925. doi: 10.1021/acs.jmedchem.1c02194
- Wang H, Xian M. Chemical methods to detect S-nitrosation. Curr Opin Chem Biol. 2011 Feb;15(1):32-7. doi: 10.1016/j.cbpa.2010.10.006
- Kettenhofen NJ, Broniowska KA, Keszler A, Zhang Y, Hogg N. Proteomic methods for analysis of S-nitrosation. J Chromatogr B Analyt Technol Biomed Life Sci. 2007 May 15;851(1-2):152-9. doi: 10.1016/j.jchromb.2007.02.035










