Traditional proteomics uses a data-dependent acquisition (DDA) strategy in which protein samples are digested into peptides, ionized and analyzed by mass spectrometry. In full scan mass spectrometry, above-noise peptide signals are selectively cleaved to produce random (MS/MS) mass spectra that can be matched to profiles in a database. Although this method is very powerful, it randomly selects peptides for cleavage, always favoring those peaks with the strongest signal. Therefore, the quantification of low abundance peptides remains challenging.
With the development and innovation of proteomics technology, a new mode of mass spectrometry data acquisition, data-independent acquisition (DIA), is coming into prominence. Compared with DDA data acquisition mode, which collects a limited number of peptide ions (usually TOP20) with high intensity in each time window in primary mass spectrometry for fragmentation analysis, DIA fragments all peptide signals in a segmented carpet mode and records the corresponding signals for analysis, which has better data reproducibility and more complete data preservation, and can achieve lossless data acquisition.
Case 1 DIA proteome for marker discovery (1)
Tau proteins play a key role in many neurodegenerative diseases, amyloid-β activation is dependent on Tau proteins and it induces Tau protein resynthesis. So, does Tau protein itself affect protein synthesis in frontotemporal dementia (FTD) and which proteins are affected?
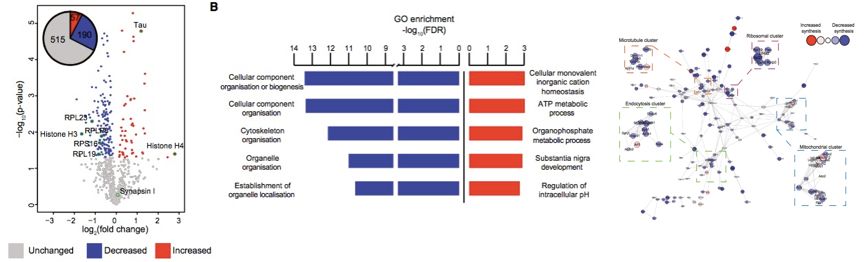
In this study, five WT and five K3 mice each were analyzed for DIA proteomics. Compared with WT, K3 mice had 57 up-regulated proteins and 190 down-regulated proteins. GO analysis revealed that down-regulated proteins in K3 mice were related to biological processes of cellular components, cytoskeleton, and tissue localization; up-regulated proteins were related to biological processes of cellular cation homeostasis, ATP metabolic processes, organophosphorus metabolic processes, nigrostriatal development, and intracellular pH regulation. Network analysis revealed mitochondrial dysfunction, tau interactions, endocytosis and ribosome-associated protein aggregation into clusters. Subsequently, WB as well as immunofluorescence were also performed to validate the results of the proteomic analysis. The final study revealed that pathological tau interferes with cellular function through dysregulation of ribosomal protein synthesis and may be an underlying mechanism of FTD.
Case 2 DIA proteome for explanatory mechanism (2)
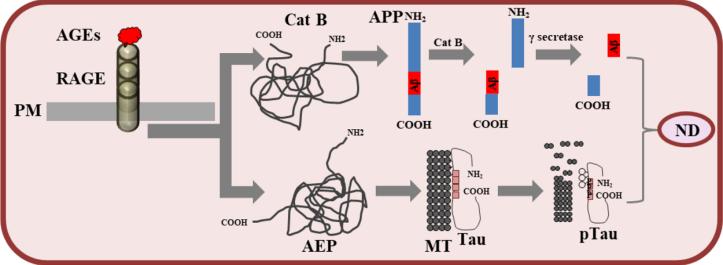
Advanced glycosylation end products (AGEs) are associated with AD pathology because AGEs induce neurodegeneration upon interaction with the receptor RAGE, but the mechanistic link between AGE-RAGE signaling and AD pathology is unclear.
Cells were treated with AGEs and changes in protein levels were detected by liquid chromatography-mass spectrometry analysis (SWATH-MS) in DIA mode, followed by WB/ELISA/luciferase to validate the mechanism as well as clinical samples Validation of the correlation revealed the relationship between advanced glycosylation end products (AGEs) and the pathology of AD. AGE-RAGE signaling may influence the pathology of AD by regulating the key AD proteins cathepsin B, asparagines endopeptidase (AEP), acid ceramidase, CD147, etc. process.
Reference
- Evans, Harrison Tudor, et al. "Decreased synthesis of ribosomal proteins in tauopathy revealed by non‐canonical amino acid labelling." The EMBO journal 38.13 (2019): e101174.
- Batkulwar, Kedar, et al. "Advanced glycation end products modulate amyloidogenic APP processing and tau phosphorylation: a mechanistic link between glycation and the development of Alzheimer's disease." ACS chemical neuroscience 9.5 (2018): 988-1000.





