I. Core Mechanism Comparison
Analysis of Functional Complementarity
Technical Comparisons in Epigenetic Profiling
Therapeutic Intervention Strategies
Frontier Research Directions
Conclusion: The Epigenetic Equilibrium Framework
People Also Ask
Epigenetics investigates heritable changes in gene activity that occur without alterations to the underlying DNA sequence. Histone post-translational modifications (PTMs) and DNA methylation represent two fundamental epigenetic mechanisms governing gene regulation. Although mechanistically distinct, these pathways frequently exhibit functional synergy, collaborating to establish sophisticated transcriptional landscapes. This review systematically examines their mechanistic distinctions and synergies, elucidating how their interplay orchestrates gene expression programs.
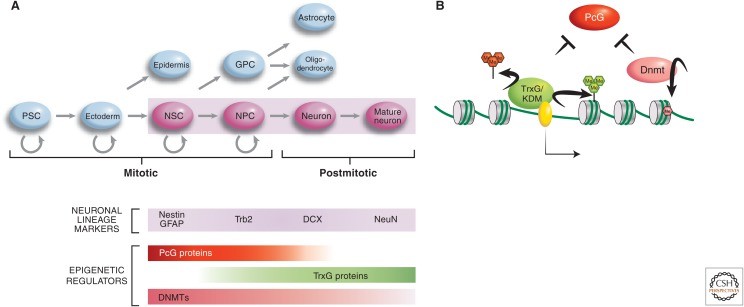 Epigenetic regulation of neurogenesis (Lomvardas S et al., 2016)
Epigenetic regulation of neurogenesis (Lomvardas S et al., 2016)
I. Core Mechanism Comparison
1. Histone PTMs
Histones package DNA into chromatin, with their chemical modifications directly altering chromatin structure and transcriptional regulation. Key PTMs include:
- Acetylation: Neutralizes histone charges, reducing chromatin compaction and facilitating transcription factor access to activate genes.
- Methylation: Exerts context-dependent effects (e.g., H3K4me3 promotes activation while H3K27me3 induces silencing).
- Phosphorylation and ubiquitination further modulate chromatin dynamics.
These modifications enable cellular adaptation to environmental stimuli through structural reorganization of chromatin.
2. DNA Methylation
This stable epigenetic mechanism involves methyl group addition to cytosine bases, predominantly at CpG-rich promoter regions. Key functions:
- Transcriptional Silencing: Methylated promoters block transcription factor binding and recruit repressive complexes (e.g., MBD proteins).
- Developmental Regulation: Governs genomic imprinting and X-chromosome inactivation.
Unlike histone PTMs, DNA methylation patterns exhibit greater stability and transgenerational inheritance.
3. Mechanistic Complementarity
Histone PTMs and DNA methylation form integrated regulatory networks despite distinct mechanisms:
| Feature | Histone PTMs | DNA Methylation |
| Chemical Target | Histone tails | Cytosine nucleotides |
| Dynamics | Rapid, reversible | Stable, heritable |
| Primary Function | Chromatin accessibility control | Long-term gene silencing |
Synergistic Interactions
- Co-localization: Repressed loci often show concurrent promoter DNA methylation and H3K27me3 deposition.
- Reciprocal Regulation:
- Methyl-CpG binding proteins recruit histone deacetylases
- H3K4me3 prevents DNA methylation establishment
- Chromatin State Control: Their combined action dynamically shifts chromatin between permissive and repressive conformations, directing developmental stage-specific transcription programs.
| Feature | Histone Modifications (PTMs) | DNA Methylation |
| Chemical Nature | Covalent modifications of histone tails (e.g., acetylation, methylation) | Methylation at the 5th carbon of cytosine in CpG islands (5mC) |
| Dynamics | Rapidly reversible (minutes to hours) | Relatively stable (hours to days) |
| Primary Function | Regulates chromatin accessibility/open state | Maintains long-term gene silencing |
| Key Enzymes | HATs/HDACs (acetylation), KMTs/KDMs (methylation) | DNMTs (methylation), TETs (demethylation) |
Analysis of Functional Complementarity
1. Three-Dimensional Genome Organization: Methylation-Guided Chromatin Architecture
Polycompartment Structural Blueprinting
- H3K27me3 Spatial Patterning:
DNA methylation directs topologically associating domain (TAD) boundary formation through:- Directional H3K27me3 enrichment/clearance → Planned heterochromatin nucleation
- Methylation-H3K27me3 axis stabilization → Chromatin compartmentalization (A/B transitions) → Prevention of non-physiological chromatin looping
Epigenetic Coordination of Enhancer-Promoter Communication
- Dynamic Enhancer Control:
DNA methylation status governs:
- H3K27ac/H3K27me3 equilibrium → Chromatin loop anchor point specification
- SETDB1 recruitment → H3K9me3 deposition at distal sites → Silencing of aberrant enhancers (e.g., proto-oncogene loci)
Biological Implications
- Spatial Gene Regulation: DNA methylation functions as a 3D genome architect, positioning chromatin interaction nodes via histone modification cascades
- Disease Pathogenesis: Methylation dysregulation → Topological collapse → Enhancer hijacking (e.g., ectopic proto-oncogene activation) (King AD et al., 2016).
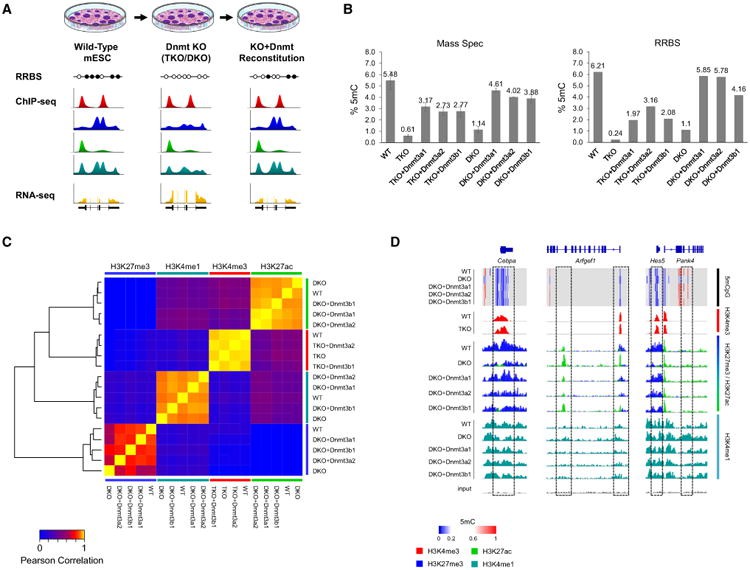 Alterations of DNA Methylation Cause Selective Genome-wide Changes in Histone Modifications (King AD et al., 2016)
Alterations of DNA Methylation Cause Selective Genome-wide Changes in Histone Modifications (King AD et al., 2016)
2. Spatial Targeting of DNA Methylation by H3K36 Methylation
- Functional Partitioning of DNMTs:
- H3K36me3 (enriched in actively transcribed gene bodies) recruits DNMT3B → maintains intragenic hypermethylation → suppresses spurious transcription initiation
- H3K36me2 (localized to intergenic/euchromatic regions) directs DNMT3A → establishes broad intergenic methylation → stabilizes genomic architecture
- Molecular Recognition Mechanisms:
- DNMT3B-PWWP domain specifically binds *SETD2-catalyzed H3K36me3*
- DNMT3A-PWWP domain preferentially interacts with *NSD1/2-generated H3K36me2*
Biological Implications of Coordinated Methylation
- Transcriptional Fidelity Maintenance: The H3K36me3-DNMT3B axis enforces gene body methylation, thereby repressing cryptic promoters and ensuring uninterrupted RNA polymerase II progression.
- Genome Topology Preservation: Through H3K36me2-DNMT3A targeting, intergenic methylation reinforces topologically associating domain (TAD) boundaries, preventing aberrant chromatin interactions (Li Y et al., 2021).
3. Coordinated Mechanisms in Heterochromatin Assembly
Spatial Targeting via H3K9 Methylation
H3K9me serves as a recruitment signal for DNMT3A/B, directing DNA methylation to specific genomic regions including centromeres and retrotransposons. Methyltransferase specialization occurs through:
- SUV39H: Recruits DNMT3B to centromeres via HP1 bridging
- SETDB1: Directly binds DNMT3A to silence retrotransposons (ERVs)
- G9A: Engages DNMT3A/B through non-catalytic mechanisms
Catalytic-Independent Recruitment by G9A
- This pathway exhibits unique characteristics:
- Recruitment occurs without methyltransferase activity (demonstrated by inactive mutants)
- Bridging complexes (HUSH/MPP8 or UHRF1) physically link G9A to DNMTs
- Enables H3K9me-independent DNA methylation establishment
Biological Significance
- Genomic Integrity Maintenance:
- Retrotransposon silencing prevents DNA fragmentation
- Centromeric heterochromatin stabilization ensures accurate chromosome segregation
- Developmental Regulation: G9A-DNMT axis-mediated methylation of imprinted genes restricts pluripotency factor expression during differentiation
Core Mechanistic Principle
H3K9 methylation designates transcriptionally silent genomic regions → DNMT3A/B executes site-specific DNA methylation → Forms synergistic "epigenetic locks" → Ensures heritable heterochromatin stability (Li Y et al., 2021)
Collaborative Regulation of Genomic Imprinting by DNA Methylation and H3K27me3
Noncanonical Imprinting (Placental Genes)
- Origin: Maternal H3K27me3 deposited in oocytes transiently marks target genes
- Transition: H3K27me3 guides de novo DNA methylation during implantation (establishing somatic differentially methylated regions, DMRs)
- Maintenance: DNA methylation enforces persistent silencing of genes (e.g., GAB1, Phf17)
Canonical Imprinting (Embryonic Genes)
- Origin: Germline-derived DNA methylation at DMRs
- Stabilization: Secondary H3K27me3 deposition reinforces silencing (e.g., Igf2), creating dual epigenetic locks
Essential Molecular Components
| Component | Functional Consequence of Loss |
| EED (PRC2) | Failed H3K27me3 inheritance → Imprinting disruption |
| DNMT3A/B | DMR establishment failure → Imprinting collapse |
Stage-specific epigenetic division of labor
| Developmental Context | Dominant Mark | Functional Role |
| Preimplantation embryos | H3K27me3 | Initiates noncanonical imprinting (transient) |
| Postimplantation soma | DNA methylation (DMRs) | Maintains long-term gene silencing |
| Placental tissue | H3K27me3 | Sustains imprinting independently of DNA methylation |
Biological Significance
- Complementary Safeguarding
- Flexible control: H3K27me3 allows dynamic regulation
- Stable inheritance: DNA methylation ensures heritable silencing
→ Synergy prevents imprinting erosion
- Developmental Adaptation
- Placental genes: H3K27me3 enables rapid response to developmental cues
- Embryonic genes: DNA methylation provides irreversible lifelong repression (Chen Z et al., 2019)
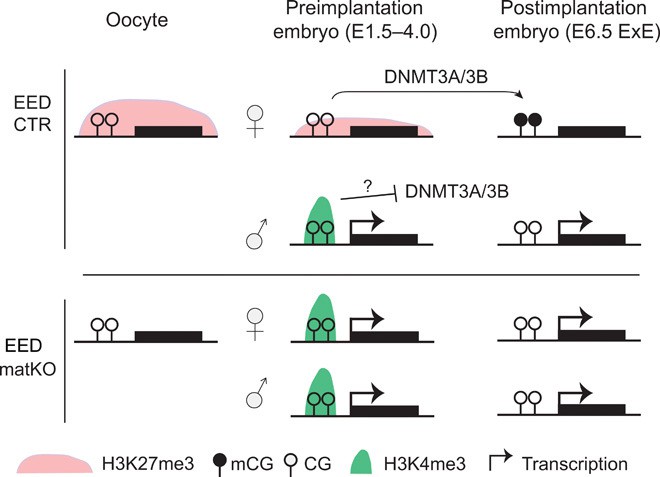 Model illustrating that maintenance of maternal H3K27me3–dependent imprinting requires somatic DMR establishment in postimplantation embryos (Chen Z et al., 2019)
Model illustrating that maintenance of maternal H3K27me3–dependent imprinting requires somatic DMR establishment in postimplantation embryos (Chen Z et al., 2019)
4. Collaborative Epigenetic Dysregulation in Tumor Pathogenesis
Core Features of Tumor Epigenetic Imbalance
- DNA Methylation Aberrations:
- CpG island hypomethylation at global genomic scales
- Focal hypermethylation at tumor suppressor promoters (e.g., IRX2)
- Histone Modification Disruptions:
- H3K4me3 depletion at transcriptionally blocked genes
- Regional H3K27me3 accumulation at aberrantly silenced loci (e.g., oncogenes)
- Key AML-Specific Findings:
Hypermethylated CpG islands exhibit:- H3K4me3 depletion + H3K4me0 accumulation
- Dominant DNA hypermethylation uncorrelated with H3K27me3
→ Challenges conventional histone-guided methylation models
Molecular Mechanisms of Synergistic Silencing
- Epigenetic Safeguard Failure:
- H3K4me3 deletion → loss of promoter protection against methylation → susceptibility to DNA hypermethylation
- H3K4me0 accumulates → opens chromatin structure → attracts DNMT3A/B binding (EG, IRX2 promoter)
- Tumor Suppressor Silencing Paradigm (IRX2 Case):
| State | Epigenetic Status | Functional Consequence |
| Normal | Unmethylated + H3K4me3+ | Hematopoietic differentiation |
| Tumor | Hypermethylated + H3K4me3- | Differentiation arrest → Leukemogenesis (Scalea S et al., 2020) |
Technical Comparisons in Epigenetic Profiling
1. Detection Methodologies
| Feature | Histone PTMs | DNA Methylation |
| Gold Standard | ChIP-seq (antibody-dependent) | Bisulfite sequencing (WGBS) |
| Single-cell Resolution | scCUT&Tag (limited coverage) | scBS-seq (high accuracy) |
| Live-cell Dynamics | Fluorescent reporters (constrained) | Methylation editors (efficient) |
2. Key Technical Challenges
- Histone PTM Limitations:
- Requires ≥10⁶ cells per assay → Precludes rare cell population analysis
- Indirect chromatin state inference (e.g., H3K27ac → accessibility)
- DNA Methylation Constraints:
- Does not directly report chromatin openness (e.g., methylated inactive vs. poised enhancers)
- Bisulfite conversion artifacts in low-input applications
Critical Implications
- Experimental Design:
- PTM studies favor pooled cell populations over single-cell approaches
- Methylation assays lack functional chromatin state resolution
- Emerging Solutions:
- Multi-omics integration (e.g., scNMT-seq) bridges chromatin accessibility with methylation states
- Engineered editors (dCas9-DNMT3A/TET1) enable real-time methylation tracking
Therapeutic Intervention Strategies
1. Targeted Epigenetic Agents
| Target | Drug | Mechanism | Clinical Application |
| DNMT | Azacitidine | Blocks DNA methyltransferases → TSG reactivation | Myelodysplastic syndromes |
| HDAC | Vorinostat | Increases histone acetylation → Chromatin opening | T-cell lymphomas |
| EZH2 | Tazemetostat | Inhibits H3K27me3 deposition → Gene desilencing | Follicular lymphoma |
2. Synergistic Therapy Breakthroughs
Neoantigen Generation Mechanism
- Epigenetic therapies reactivate endogenous retroviral elements (ERVs) through:
- DNMT inhibitors (e.g., decitabine) and HDAC inhibitors (e.g., vorinostat)
- ERV promoter derepression
- Production of novel transcripts (treatment-induced novel polyadenylated transcripts, TINPATs)
- Immunogenicity Validation
- Identification: 45 treatment-induced neopeptides (t-neopeptides) characterized
- Presentation: Immunopeptidomics confirmed HLA-mediated t-neopeptide display
- Response: Demonstrated T-cell activation in vitro
- Clinical Relevance
- t-neopeptides detected in acute myeloid leukemia (AML) patients following decitabine treatment
- Correlates with therapeutic response in epigenetic therapy trials
- Therapeutic Applications
| Approach | Limitation | Applicable Cancers |
| Conventional neoantigens | Requires high mutational burden | Melanoma (high-mutation) |
| t-neopeptide strategy | Epigenetically inducible | Leukemia, ovarian (low-mutation) |
- Combination therapy: Apparent Drug Pretreatment (72 H)→ PD-1/CTLA -4 inhibitors → objective response rate (Orr) of 60% in clinical trials (single dose < 20%)
- Personalised vaccine development: Design of personalized t-neopeptide vaccines based on patient-specific TINPATs →(complete regression of preclinical model tumors) (Goyal A et al., 2023)
Synergistic Mechanisms in PTCL Epigenetic Therapy
1. Dual-Pathway Epigenetic Blockade
| Agent | Primary Mechanism | Functional Outcome |
| Azacitidine (DNMT inhibitor) |
DNA hypomethylation → Tumor suppressor gene reactivation (e.g., TET2, TP53) |
Restoration of tumor suppressive functions |
| Romidepsin (HDAC inhibitor) |
↑ H3K27ac → Chromatin remodeling | Activation of apoptotic/differentiation genes (e.g., BCL2L11) |
- Cross-Mechanistic Synergy:
- DNMT inhibitor chain of action: Inhibition of DNA methylation → chromatin relaxation → enhancement of histone modification efficiency by HDAC inhibitors
- HDAC inhibitor action chain: Inhibition of histone deacetylation → activation of gene transcription → amplification of DNMT inhibitor-induced demethylation
Core features: Bi-directional cross-amplification-apparent dual-pathway blockade forms a"Positive feedback loop" that synergistically activates tumor suppressor genes and induces an immune response.
2. Biomarker-Stratified Therapeutic Response
| Predictive Factor | Response Profile | Molecular Basis |
| TFH-PTCL subtype | 80% sensitivity to dual therapy | Synergistic tumor suppressor activation |
| *TET2/DNMT3A/IDH2* mutations | 2× elevated ORR | Enhanced epigenetic vulnerability |
| ERV-derived neoantigens | T-cell activation marker | Drug-induced immunogenic presentation |
- Clinical Validation:
- TFH-PTCL patients: 67% complete response rate (vs. <40% other subtypes)
- Mutation carriers: 2-fold increase in objective response rate (Falchi L et al., 2021)
Frontier Research Directions
1. Phase Separation Regulation
H3K27me3-driven liquid-liquid phase separation facilitates heterochromatin compaction, coordinating DNA methylation to stabilize condensed chromatin architecture.
2. Transgenerational Epigenetics
- Spermatozoa transmit environmental memories through dual mechanisms:
- DNA methylation patterning
- Retained histone modifications (H3K4me3/H3K27me3) at key developmental loci
3. AI-Driven Prediction
- Deep learning frameworks (e.g., DeepSEA) integrate histone PTM and methylation profiles to:
→ Identify disease-associated genes
→ Predict pathological risk trajectories
Conclusion: The Epigenetic Equilibrium Framework
Dual-Layer Regulatory System
| Component | Functional Role | Biological Significance |
| Histone PTMs | Rapid chromatin state mapping | Adaptive response to transient stimuli |
| DNA Methylation | Stable epigenetic memory consolidation | Developmental program fidelity |
Pathological Implications & Therapeutic Outlook
- Disease Pathogenesis: Dysregulation between rapid PTM responses and stable methylation underlies cancer/neurodegeneration
- Reset Therapeutic Strategies:
- Develop dual-target epigenetic editors
- Achieve precise reprogramming of malignant/degenerative cells
- Future Objectives:
- Engineering tools for simultaneous histone-DNA modification
- Restoring homeostatic balance in pathological epigenomes
Understanding histone ptms in neurobiology can be consulted "Histone PTMs in Neurobiology: From Memory to Neurodegeneration".
For more research on histone PTM biomarkers see "Histone PTMs as Biomarkers: Opportunities and Limitations for Translational Research".
People Also Ask
What is the difference between DNA methylation and histone methylation?
DNA methylation directly modifies the DNA sequence, while histone methylation alters the structure of histone proteins, which in turn affects how DNA is packaged and accessed by the transcription machinery.
Is DNA methylation the same as epigenetics?
Epigenetic modifications can be maintained from cell to cell as cells divide and, in some cases, can be inherited through the generations. A common type of epigenetic modification is called DNA methylation.
What is the effect of histone modification and methylation on DNA packaging?
The effect on DNA packaging would be that in histone acetylation, chromatin is modified into a more relaxed structure that permits transcription while in DNA methylation, the DNA activity is modified without the DNA sequence being changed.
What are the three major epigenetic mechanisms?
Three different epigenetic mechanisms have been identified: DNA methylation, histone modification, and non-coding RNA (ncRNA)-associated gene silencing.
References
- Lomvardas S, Maniatis T. "Histone and DNA Modifications as Regulators of Neuronal Development and Function." Cold Spring Harb Perspect Biol. 2016 Jul 1;8(7):a024208. doi: 10.1101/cshperspect.a024208
- King AD, Huang K, Rubbi L, Liu S, Wang CY, Wang Y, Pellegrini M, Fan G. "Reversible Regulation of Promoter and Enhancer Histone Landscape by DNA Methylation in Mouse Embryonic Stem Cells." Cell Rep. 2016 Sep 27;17(1):289-302. doi: 10.1016/j.celrep.2016.08.083
- Li Y, Chen X, Lu C. "The interplay between DNA and histone methylation: molecular mechanisms and disease implications." EMBO Rep. 2021 May 5;22(5):e51803. doi: 10.15252/embr.202051803
- Chen Z, Yin Q, Inoue A, Zhang C, Zhang Y. "Allelic H3K27me3 to allelic DNA methylation switch maintains noncanonical imprinting in extraembryonic cells." Sci Adv. 2019 Dec 20;5(12):eaay7246. doi: 10.1126/sciadv.aay7246
- Scalea S, Maresca C, Catalanotto C, Marino R, Cogoni C, Reale A, Zampieri M, Zardo G. "Modifications of H3K4 methylation levels are associated with DNA hypermethylation in acute myeloid leukemia." FEBS J. 2020 Mar;287(6):1155-1175. doi: 10.1111/febs.15086
- Goyal A, Bauer J, Hey J, Papageorgiou DN, Stepanova E, Daskalakis M, Scheid J, Dubbelaar M, Klimovich B, Schwarz D, Märklin M, Roerden M, Lin YY, Ma T, Mücke O, Rammensee HG, Lübbert M, Loayza-Puch F, Krijgsveld J, Walz JS, Plass C. "DNMT and HDAC inhibition induces immunogenic neoantigens from human endogenous retroviral element-derived transcripts." Nat Commun. 2023 Oct 23;14(1):6731. doi: 10.1038/s41467-023-42417-w
- Falchi L, Ma H, Klein S, Lue JK, Montanari F, Marchi E, Deng C, Kim HA, Rada A, Jacob AT, Kinahan C, Francescone MM, Soderquist CR, Park DC, Bhagat G, Nandakumar R, Menezes D, Scotto L, Sokol L, Shustov AR, O'Connor OA. "Combined oral 5-azacytidine and romidepsin are highly effective in patients with PTCL: a multicenter phase 2 study." Blood. 2021 Apr 22;137(16):2161-2170. doi: 10.1182/blood.2020009004


